|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
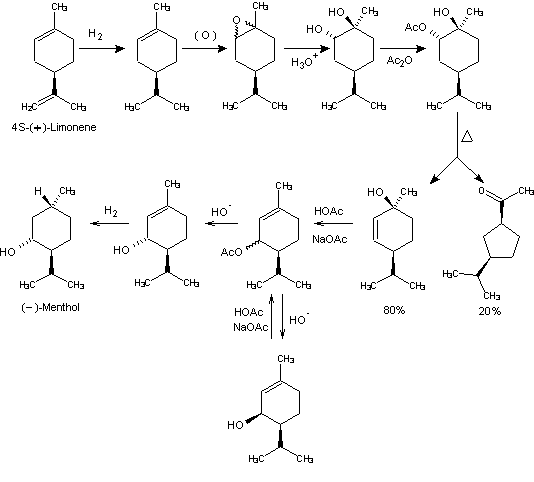
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. (+)-Limonene is the abundant by-product terpene derived from steam distilling orange and lemon peels. (+)-Limonene of high optical purity was hydrogenated to (+)-1-menthene over a Raney-Ni catalyst (97% yield). Epoxidation gives 1-menthene epoxides which on hydrolysis yields mainly (+)-l-hydroxyneocarvomenthol. This is acetylated to (+)-l-hydroxyneocarvomenthyl acetate. Pyrolysis of (+)-l-hydroxyneocarvomenthyl acetate gave a mixture of 8 parts of (-)-trans-2-men- thene-l-ol and 2 parts of the novel ring contraction product, 3-isopropylcyclopentyl methyl ketone. Although the pyrolysis products can be purified at this stage, it is more practical on a large scale to subject the crude pyrolysate to solvolysis in acetic acid/sodium acteate wherein the 2-menthene-1-ol is converted to a mixture of the piperityl acetates through allylic substitution-rearrangement (42% cis/58% trans). Inasmuch as the isomeric cis and trans piperityl acetates are separable only with difficulty by fractional distillation, it is most convenient to take the crude mixtures of acetic acid solvolyzed crude pyrolyzate and hydrolyze the total mixture with base to give the corresponding mixed (+)-cis and (-)-trans-piperitols. These isomeric alcohols are readily separable by distillation along with the 3-isopropylcyclopentyl methyl ketone formed during the pyrolysis step. This latter ketone, although of no further interest in the menthol synthesis, is interesting from several other standpoints. First, reduction to 1-(3-isopropylcyclopentyl)-l-ethanol provides the interesting linalool-muguet floral type perfumery material reported by Eschinasi. Having separated the (+)-cis and, (-)-trans piperitols,
there are several alternative courses of action for
obtaining (-)-menthol. First, "selective" hydrogenation of
(+)-cis-piperitol stereospecifically to (+)-neomentbol would
"fix" the absolute configuration at C-1 so that upon
equilibration of the C-3, C-4 asymmetric centers,
(-)-menthol would be the predominant product. Unfortunately,
in all cases some (-)-neoisomentbol was produced and because
this alcohol possesses the opposite absolute configuration
at C-1, upon isomerization it would yield (+)-menthol. For
this reason we decided to recycle the (+)-cis piperitol back
into the solvolysis system used on the
(-)-trans-2-menthene-l-ol for production of equilibrated
piperityl acetates. In this way the cis-piperitol was
converted to a 42% Cis/58% trans mixture from which
additional Having abandoned any real consideration of reducing cis-piperitol to menthol isomers, we now undertook a brief study on the direct reduction of (-)-trans-piperitol to (-)-menthol. It shortly became apparent that 5% Pd/C in ethanol would provide 75% (-)-menthol and 25% (-)-isomentbol. Fortunately, these two isomers are separable by efficient fractional distillation and (-)-menthol of high purity was readily obtained. Since the (-)isomenthol formed is of the opposite optical series, the only utility this material has is for production of mentbone (by oxidation) or racemic menthol via isomerization techniques. 1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993).
|
J.C. Leffingwell, & R.E. Shackelford
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY