|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. The 2d Glidco synthesis utilized
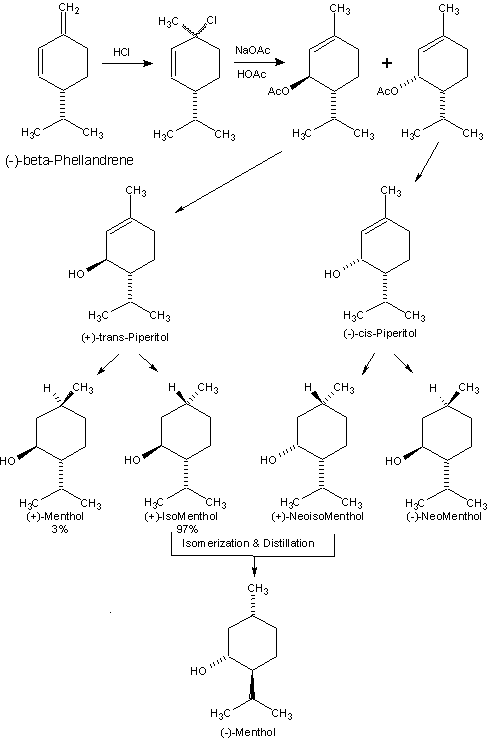
(-)-beta-Phellandrene which is available in high optical
purity from the turpentine of the Lodgepole pine (Pinus
contorta) where it is the major constituent. The same
stereochemical complexities that plague menthol production
from (-)-piperitone also are encountered if
(-)-alpha-phellandrene (from Eucalyptus dives) or
beta-phellandrene are used as the raw materials. Addition of
hydrogen chloride to either (-)-beta-phellandrene or
beta-Phellandrene from beta-Pinene. The overall yield of turpentine high in phellandrene is considerably less than the high yielding Longleaf pine (Pinus palustras, who's composition contains about 21-22% (-)-beta-pinene of high optical purity ), e.g. 0.54 gallons/ton dry wood vs. 4.06 gallons/ton dry wood. Kane and Traynor found an elegant way to prepare (-)-beta-phellandrene by the pyrolysis of para-menth-1-ene-7-sulfonate (which is formed by heating an aqueous reaction mixture of beta-pinene and a bisulfite salt under free radical conditions)(4,5). The optical purity of the products is maintained through this process.
1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993). 3. Kane; Bernard J.; Irving; Karen E.; Bledsoe, Jr.; James O.; Canova; Levy A., Hydrogenation of cyclic unsaturated compounds, U.S. Patent 4,058,572, November 15, 1977. 4. Kane; Bernard J.; Traynor; Sean G., Preparation of para-menth-1-ene-7-sulfonate salts and corresponding acids . U.S. Patent 4,224,240, et al. September 23, 1980. 5. Hirschy; Linda M.; Kane; Bernard J.; Traynor; Sean G., Preparation of beta-phellandrene, U.S. Patent 4,136,126, January 23, 1979. |
(B.J. Kane)
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY