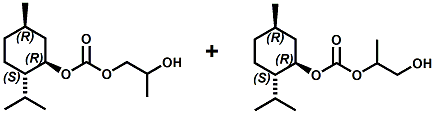|
Leffingwell &
Associates
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com 
|
|
Cool without Menthol |
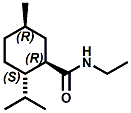
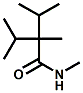
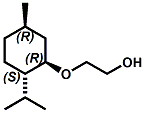
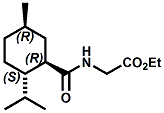
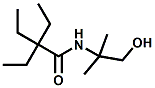
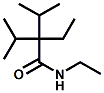
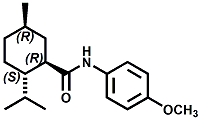
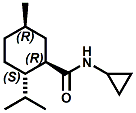
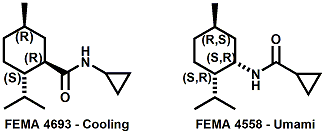
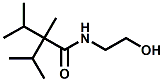
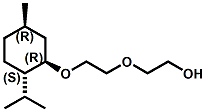
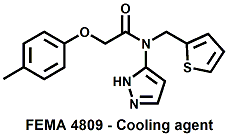
and Cooling Compounds as Insect Repellents By John C. Leffingwell, Ph.D. (Updated June 8, 2018) Cool without Menthol Over the last 30 years a considerable number of compounds have been synthesized and evaluated for the physiological sensation of "cooling". In the 1970's Wilkinson Sword Ltd. conducted an extensive research program under the company leadership of Roy Randolph (who interestingly was my Aunt Joyce's husband until his death). During this period Hugh R. Watson, David G. Rowsell and co-workers designed and evaluated about 1200 compounds for their cooling activity1-18. The interest in such compounds relates to cooling sensation without the minty and volatile side effects of menthol such as eye irritation from aftershave lotions, etc. The impact of the Wilkinson-Sword research on cooling compounds and cooling receptors has recently been reviewed (John C. Leffingwell & David G. Rowsell, Wilkinson Sword Cooling Compounds: From the Beginning to Now, Perfumer & Flavorist, Vol. 39, 3 [March Issue], 2014, pp. 34-43). Of these original Wilkinson Sword compounds, eight have been successfully commercialized - WS-23 (2-Isopropyl-N,2,3-trimethylbutyramide), FEMA 3804; WS-3 (N-Ethyl-p-menthane-3-carboxamide), FEMA 3455; WS-5 [Ethyl 3-(p-menthane-3-carboxamido)acetate], FEMA 4309; WS-12 (1R,2S,5R)-N-(4-Methoxyphenyl)-p-menthanecarboxamide, FEMA 4681; WS-27 (N-Ethyl-2,2-diisopropylbutanamide), FEMA 4557; N-Cyclopropyl-5-methyl-2-isopropylcyclohexanecarboxamide, FEMA 4693, WS-116 (N-(1,1-Dimethyl-2-hydroxyethyl)-2,2-diethylbutanamide), FEMA 4603 and Menthoxyethanol, FEMA 4154. FEMA 4154 was first patented by Wilkinson-Sword as a cooling agent in German Patent DE2203947 (1972). Four more related carboxamides have received FEMA GRAS status (FEMA GRAS List 24) - N-(4-cyanomethylphenyl)-p-menthanecarboxamide, FEMA 4496; N-(2-(Pyridin-2-yl)ethyl)-3-p-menthanecarboxamide, FEMA 4549; N-(2-Hydroxyethyl)-2-isopropyl-2,3-dimethylbutanamide, FEMA 4602 and (also N-(4-(carbamoylmethyl)phenyl)-menthylcarboxamide, FEMA 4684 in FEMA GRAS List 25). In the case of WS-3, the suppliers (Symrise (formerly Renessenz), Givaudan, Frutarom [as Framidice 3] , Natural-Advantage, Jiangsu Grand Chem & Qarôma [as ICE 3000], ) often do not specify optical purity, but Watson et .al. indicate, in general, that compounds in the all equitorial form (like menthol) are preferred and that WS-3 with the1R,3R,4S form (like (-)-menthol) should be optimal in cooling aspects. However, we are unaware of comparative studies that provide specific cooling properties of the various enantiomers. As WS-23 has no chiral center this compound (supplied by (Symrise (formerly Renessenz), AromaLink Penta, Shanghai Vigen, Jiangsu Grand Chem & Qarôma ) has only one form. WS-5 (FEMA 4309), which, until recently was the "coldest" of commercially available "coolants" is available from (Symrise (formerly Renessenz) & Qarôma. N-Ethyl-2,2-diisopropylbutanamide, FEMA 4557, N-(2-Hydroxyethyl)-2-isopropyl-2,3-dimethylbutanamide, FEMA 4602 and N-(1,1-Dimethyl-2-hydroxyethyl)-2,2-diethylbutanamide, FEMA 4603 are also available from Qarôma. N-(4-cyanomethylphenyl)-p-menthanecarboxamide, FEMA 4496 and N-(2-(Pyridin-2-yl)ethyl)-3-p-menthanecarboxamide, FEMA 4549, are products of Givaudan.WS-12 is available from (Symrise (formerly Renessenz) and Qarôma. In 2011, on FEMA GRAS List 25, the following three carboxamide cooling agents were added - (1R,2S,5R)-N-(4-Methoxyphenyl)-p-menthanecarboxamide (WS-12), FEMA 4681; (2S,5R)-N-[4-(2-Amino-2-oxoethyl)phenyl]-p-menthanecarboxamide, FEMA 4684; and N-Cyclopropyl-5-methyl-2-isopropylcyclohexanecarbonecarboxamide, FEMA 4693; Other cooling agents added were 2-[(2-p-Menthoxy)ethoxy]ethanol, FEMA 4718, as well as a material that adds a refreshing effect (2,6-Diethyl-5-isopropyl-2-methyltetrahydropyran, FEMA 4680) and a TRPV1 antagonist that inhibits heat sensation and thus enhances cooling agents (trans-4-tert-Butylcyclohexanol, FEMA 4724). In July 2014, the Senomyx compound, 2-(p-tolyloxy)-N-(1H-pyrazol-5-yl)-N-((thiophen-2-yl)methyl)acetamide, FEMA 4809 was added to the list. The 2013 Japanese Flavor list (updated as of April 2015) includes 15 of the 32 FEMA GRAS cooling compounds from the FEMA lists thruough GRAS List 26. These are those with FEMA numbers 2962, 3748, 3784, 3805, 3806, 3008, 3810, 3849, 4006, 4053, 4154, 4308, 4309, 4604, 4718 and 4681 shown in the tables below. While normally aliphatic and aromatic amides are not permitted as flavorants in Japan, the latest list includes the coolants WS-5 (classified under esters) and WS-12 (classified under phenol ethers).
OTHER IMPORTANT GRAS COOLANTS
Renessenz describes WS-3 as white crystalline and almost odorless that is characterized by a high cooling activity with no side effects such as burning, stinging or tingling sensations. Its chief use is as a coolant in medicinal preparations, oral care products and confectionery products. Givaudan indicates this chemical is effective in combination with mint oils. It gives more impact, freshness and long-lasting flavour. The taste threshold is 200 ppb (by placing impregnated paper strip in the mouth). Mosciano, et. al. (2000) describes the Odor @ 10% as "Virtually odorless being slightly alcoholic and cooling" and the Taste @ 10-100 ppm as "Intense lingering cooling Trigeminal effect. The cooling sensation slowly but steadily grows to a lingering cooling mouth feel with a slightly camphoraceous and minty character." WS-5 is known to be significantly cooler than WS-3. In US Patent No. 7189760 by Mark Erman, et al. (March 13, 2007) they indicate that, for "highly purified (1R,2S,5R)-WS-5", the perceived cooling is about 2.5-3.0 times stronger than WS-3. See also Mark Erman, Progress in Physiological Cooling Agents, Perfumer & Flavorist, Vol. 29, No. 8, pp. 34-50 (2004). Renessenz indicates for WS-12 - WS-12 offers one of the strongest initial cooling impacts and a significantly longer lasting effect compared with conventional coolants such as WS-3, WS-23 and WS-5. It has potential for use in mint flavors in oral care applications, mouthwash, confectionery and chewing gum, depending on dosage levels. In addition, it can be used at low levels to impart freshness into berry, citrus and other fruit flavors in a variety of applications. In the US Patent 7,030,273 by Hong Sun, entitled Novel Compounds with Physiological Cooling Effect (April, 18, 2006) assigned to Qarôma, a new series of compounds, many with cooling intensity equal to or greater than WS-23 have been disclosed. In particular, N-(2-hydroxyethyl)-2,3-dimethyl-2-isopropylbutyramide (now FEMA 4602) when used in conjunction with other cooling agents (e.g. WS-3) was found to provide suprior cooling and flavor properties in confections and chewing gum. See United States Patent Application 20080293821 A1 (Nov.27, 2008) and WIPO Patent Application WO2008039522 ((Mar. 4, 2008). See also United States Patent Application 20080293821 (Nov. 27, 2008)
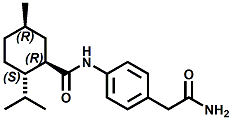
In United States Patent Application 20080038386 by A. Moza, S.E. Rohde, J.C. Leffingwell, D. Leffingwell, entitled "Use of a liquid cooling sensate to provide a pungent, warming or tingling sensate composition" (Feb. 14, 2008) it is mentioned that N-(1,1-Dimethyl-2-hydroxyethyl)2,2-diethylbutanamide (now FEMA 4603) and/or N-(2-hydroxyethyl)-2,3-dimethyl-2-isopropylbutyramide (now FEMA 4602) in conjunction with warming agents enhances the warming sensation. Two more of the original Wilkinson Sword carboxamides were added in the GRAS 25 list. WS-12 ((1R,2S,5R)-N-(4-Methoxyphenyl)-p-menthanecarboxamide) which was reported to have a cooling strength of 150 as compared to (-)-menthol at 100). Data from: Watson, Hems, Rowsell & Spring, J. Soc. Cosmet. Chem., 1978, pp. 185-200. More recently, the cooling strength of WS-12 was reported to have the same isointensity as (-)-menthol (Stefan M. Furrer, Jay P. Slack, Scott T. McCluskey, Ioana M. Ungureanu, Andrew T. Daniher, Guillaume Blancher, Karen Bell & Pablo Krawec, Lucienne Cole, Kim Gray, New Developments in the Chemistry of Cooling Compounds, Chem. Percept., 2008, 1,119-126). Renessenz indicates that, unlike menthol, WS-12 is virtually nonvolatile, odorless and tasteless. WS-12 offers one of the strongest initial cooling impacts and a significantly longer lasting effect compared with conventional coolants such as WS-3, WS-23 and WS-5. It has potential for use in mint flavors in oral care applications, mouthwash, confectionery and chewing gum, depending on dosage levels. In addition, it can be used at low levels to impart freshness into berry, citrus and other fruit flavors in a variety of applications. The second WS compound added was N-Cyclopropyl-5-methyl-2-isopropylcyclohexanecarbonecarboxamide, FEMA 4693. In United States Patent 4150052, this latter compound was reported as having an oral cooling threshold of 0.5 micrograms as compared to 0.25 micrograms for (-)-menthol. This indicates that FEMA 4693 has about 50% the cooling intensity of menthol. (2S,5R)-N-[4-(2-Amino-2-oxoethyl)phenyl]-p-menthanecarboxamide, FEMA 4684, was the other carboxamide coolant added to the GRAS 25 list. This material is the subject of United States Patent Application 20100272655 (October 28, 2010) entitled Menthylcarboxamides and Their Use as Cooling Agents by K.A. Bardsley et al. assigned to IFF. At the level of 10 ppm in water this material contributes moderate throat and mouth cooling that lingers with some bitter notes in background. Cooling lasts in the breath for 30 minutes. At the level of 1 ppm in water it was clean with slight cooling up front that builds with time to moderate level. Cooling lasted for 30 minutes. Other commercial cooling chemicals include the Menthone glycerol ketal19 (sold as Frescolat® MGA by Haarmann & Reimer). Both the racemic and leavo-forms appear on the FEMA GRAS list but the leavo-form appears to be the item of commerce. (-)-Menthyl lactate (sold as Frescolat® ML by Haarmann & Reimer ) is faintly minty in odor and virtually tasteless, with a pleasant, long-lasting cooling effect.
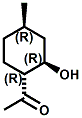
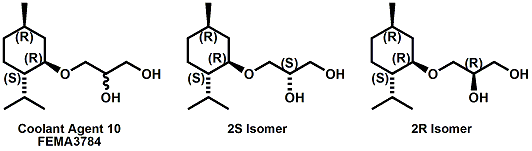
(-)-Menthoxypropane-1,2-diol20 (sold as Coolant Agent 10 by Takasago International) is another commercial cooling agent. Takasago reports that the threshold (in mouth) is 1 ppm (about 20-100% that of menthol) and that the time of cold feeling maintenance is 20-25 minutes for a 100 ppm solution ( about twice that of menthol). While the cooling strength of Cooling agent 10 is accepted as being about 20-25% that of menthol, it is also noted that "in a Vaseline ointment, 3-(l-menthoxy)propane-1,2-diol shows a cool feeling 2.0 to 2.5 times stronger than that of l-menthol".25 It should also be noted that the cool-feeling intensity of the (2S) isomer is from 2 to 3 times superior to that of the (2R) isomer and is from 1.5 to 2 times superior to that of the racemic modification.28
A related material, 3-(l-Menthoxy)-2-methylpropane-1,2-diol, appears on the FEMA GRAS list as FEMA 3849. Although Isopulegol has previously been mentioned in the literature as having a cooling sensation, normally associated with a Minty, herbaceous, bitter-sweet odor and taste, Takeshi Yamamoto of Takasago has recently found21 that highly purified (-)-Isopulegol (in excess of 99.7% enantiomeric purity) is odorless and that the optically active form imparts a feeling of freshness, crispness, and coolness to citrus type fragrances. Sensate mixtures containing (-)-Isopulegol, menthyl lactate and menthoxypropanediol have been patented22 for cosmetics. (-)-Isopulegol is sold under the name "Coolact P® " by Takasago International.
Takasago has patented the cis & trans p-Menthane-3,8-diols24 (PMD38 or Coolact 38D® ) as cooling agents25. p-Menthane diol (PMD), along with nootkatone and Patchouli alcohol are important natural / nature identical flavor & fragrance constituents that are effective against mosquitos and other insects. Takasago's PMD (p-Menthane diol) is already one of the few such CDC recommended insect repellents that are safe and effective for use by pregnant and breastfeeding women. Although numerous other compounds (e.g., 2,3-dihydroxy-p-menthane, 3,3,5-trimethylcyclohexanone glycerol ketal) are mentioned in the literature and patents as having intrinsic cooling properties, the ones described here are those that possess very little or no odor and are sold in commerce. In the case of Questice® (menthyl pyrrolidone carboxylate), the cooling property is considered not to be intrinsic but arises from enzymatic hydrolysis to menthol. Questice received FEMA GRAS status in 2005. In March 2002, a Firmenich patent discloses the use of (1R,3R,4S)-3-menthyl-3,6-dioxaheptanoate, (1R,2S,5R)-3-menthyl methoxyacetate, (1R,2S,5R)-3-menthyl 3,6,9-trioxadecanoate, (1R,2S,5R)-3-menthyl 3.6,9-trioxadecanoate, (1R,2S,5R)-3-menthyl (2-hydroxyethoxy)acetate & (1R,2S,5R)-menthyl 11-hydroxy-3,6,9-trioxaundecanoate as cooling sensate compounds.
In 2001, Firmenich also patented Cubebol as a cooling and refreshing agent.27 This is FEMA 4497.
Further, in early 2004, T. Hasegawa Co., Ltd. patented a new series of cooling compounds based on alkyloxy amides in the p-menthane series29. In 2005, Givaudan patented a series of new p-menthane carboxamides - one of which [ N-(4-cyanomethylphenyl) p-menthanecarboxamide ] (now FEMA 4496) is claimed to be 10 times cooler than menthol31.
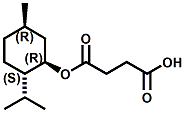
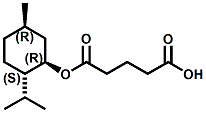
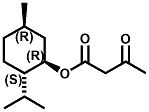
Also in 2005, IFF patented32 a series of menthyl half acid ester derivatives, one of which is N,N-Dimethyl menthyl succinamide (or more correctly 2-isopropyl-5-methylcyclohexyl 4-(dimethylamino)-4-oxobutanoate). This compound also received FEMA GRAS status (FEMA No. 4230) in 2005. Sensory evaluation indicated: Cooling and refreshing on tongue, palate and front gums; fruity flavor with estery topnotes and sour undertones. Cooling onset time 25 seconds. Cooling duration time 11.25 minutes. In a chewing gum at 0.2% it increased sweetness and exhibited a pleasant and substantive cooling effect on the tongue and roof of the mouth. In November 2005, Qarôma announced the development of a new liquid cooling sensate called ICE 1500 (see U.S. Patent Application No. 2007/0059417 (March 15,2007) and U.S. Patent Application No. 2007/0048424 (March 1, 2007) ). This cooling sensate is a Eutectic mixture of ICE 1000 (WS-23) and ICE 3000 (WS-3), both of which are GRAS cooling compounds. The ICE 1500 remains liquid even at temperatures below -20o C. Further, ICE 1500 combines the instant cooling sensations of ICE 1000 with the gradual and smooth cooling sensation of ICE 3000. It also eliminates the dust problem associated with the handling of both the ICE 1000 and ICE 3000. In flavor applications, ICE 1500 provides saltiness and/or flavor enhancement of about 20-30% in a wide variety of foodstuffs such as salsas, salad dressings and marinades, margarine, soups & bouillons, as well as alcoholic beverages, when used at levels where the cooling sensation is imperceptible or barely perceptible. To download the full press release Click HERE. See also the United States Patent Application 20050265930 by Erman, et. al., (December 1, 2005) entitled Physiological cooling compositions which describes similar liquid cooling compositions. Related to the two aforementioned Qaroma patents is the discovery that other cooling compounds such as N-(4-cyanomethylphenyl) p-menthanecarboxamide (FEMA 4496), N-(2-pyridin-2-ylethyl) p-menthanecarboxamide (FEMA 4549), Menthyl lactate (FEMA 3748), Menthol, WS-3, WS-23 and (-)-Menthoxypropane-1,2-diol (TK-10) increase saltiness at levels (0.005-0.05 ppm), significantly below their cooling threshold. See Kimberley Gray, Gregory L. Yep & Robert G. Eilerman, WIPO Patent Application WO/2008/148234 (December 11, 2008) entitled Salt Enhancement. In 2006, Firmenich filed patent applications for the use of 6-isopropyl-3,9-dimethyl-1,4-dioxaspiro[4.5]decan-2-one, in the form of any one of its isomers or of a mixture thereof as having an enhanced refreshing cooling effect (with some minty, fruity notes). See United States Patent Application 20060249167 (November 9, 2006). 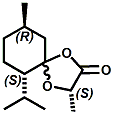
6-isopropyl-3,9-dimethyl-1,4-dioxaspiro[4.5]decan-2-one
=
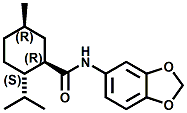
N-benzo[1,3] dioxol-5-yl-3-p-menthanecarboxamide In 2006, Givaudan patented a series of new menthane carboxamides (including N-benzo[1,3] dioxol-5-yl-3-p-menthanecarboxamide, N-benzooxazol-4-yl-3-p-menthanecarboxamide, N-4-([1,2,4]triazol-l-yl)-phenyl-3-p-menthanecarboxamide & N-4-(pyrazol-l -yl)-phenyl-3-p-menthanecarboxamide) which had cooling strengths 100X stronger than menthol. See COLE, LUCIENNE; FURRER, STEFAN MICHAEL; GALOPIN, CHRISTOPHE; KRAWEC, PABLO VICTOR; MASUR, ADAM; AND SLACK, JAY PATRICK; WO Patent No. 2006092074 (Sept. 8, 2006) assigned to Givaudan.
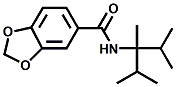
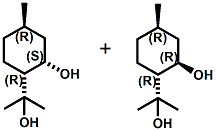
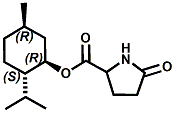
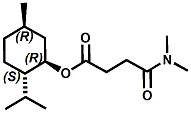
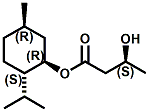
N-(1-isopropyl-1,2-dimethylpropyl)-1,3-benzodioxole-5-carboxamide Givaudan has also developed a series of aryl carboxamide analogs (with the reversed amide configuration), many with cooling intensities equal to or greater than WS-23. For example, N-(1-isopropyl-1,2-dimethylpropyl)-1,3-benzodioxole-5-carboxamide is about 2.2 times more cooling as compared to 2 ppm of menthol. See Cole L, Furrer SM, Galopin C. Cooling Compounds. PCT WO 2006/099762, 2006, Sept. 28. 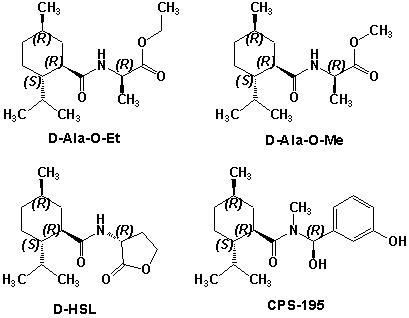 In 2006 & 2007, Wei has shown that several materials related to WS-5 possess strong cooling with remarkable cooling longevity. For example, the methyl and ethyl ester analogs of WS-5 (referred to as D-Ala-O-Me and D-Ala-O-Et, respectively) produced from D-alanine (rather than glycine). Similarly, when D-homoserine lactone is employed the resultant compound is N-( R)-2-oxotetrahydrofuran-3-y1-(1R,2S,5R)-p-menthane-3-carboxamide (referred to as "D-HSL") which also is a potent long lasting coolant. By combining suitable sympathomimetic amine drugs that act as alpha-adrenergic receptor agonists to form the corresponding p-menthane carboxamides, Wei found certain compounds (such as L-Phenylephrine p-menthane carboxamide, referred to as CPS-195) that were effective as long lasting coolants and possessed additional therapeutic properties. 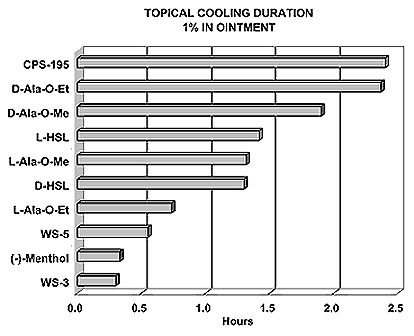 The cooling duration of a number these, applied to the skin as a 1% wt/vol in a petrolatum based ointment, vs. leading coolants is shown in the figure above. See Wei E.T. N-Alkylcarbonyl-Amino Acid Ester and N-Alkylcarbonyl-Amino Lactone Compounds and Their Use. PCT WO 2006103401, 2006, Oct 5 and Wei E.T. N-arylshydroxyalkylidene-carboxamide compositions and methods. U.S. Patent Application 20070155755, 2007, July 5.
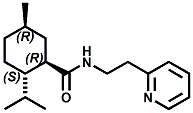
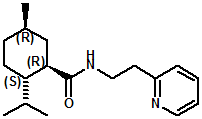
 Ratio: ~65:30 In 2007, Sergey Selifonov (Aromagen) patented a novel mixture of 2,2,5,6,6-pentamethyl-2,3,6,6a-tetrahydropentalen-3a(1H)-ol and 5-(2-hydroxy-2-methylpropyl)-3,4,4-trimethylcyclopent-2-en-1-one which had a strong long lasting cooling sensation and a faint menthol like odor. See United States Patent Application No.20070274928 (November 29, 2007). In 2007, Bell et. al. (Givaudan) disclosed a series of pyridinalkyl)yclohexanecarboxamides such as (1R,2S,5R)- 2-isopropyl-5-methyl-N-(2-(pyridin-2-yl)ethyl)cyclohexanecarboxamide (Now FEMA 4549) and (2S,5R)- 2-isopropyl-5-methyl-N-(2-(pyridin-4-yl)ethyl)cyclohexanecarboxamide that were effective cooling agents. For example, (1R,2S,5R)- 2-isopropyl-5-methyl-N-(2-(pyridin-2-yl)ethyl)cyclohexanecarboxamide had a cooling intensisty at 0.02 ppm comparable to 2.0 ppm menthol and a cooling duration about 70% longer. See Bell et. al., WO2007019719 (Feb. 22, 2007). Stefan M. Furrer, Jay P. Slack, Scott T. McCluskey, Ioana M. Ungureanu, Andrew T. Daniher, Guillaume Blancher, Karen Bell, Pablo Krawec, Lucienne Cole, Kim Gray, New Developments in the Chemistry of Cooling Compounds, Chem. Percept. (2008) 1:119–126 - indicate that 0.05 ppm of (1R,2S,5R)- 2-isopropyl-5-methyl-N-(2-(pyridin-2-yl)ethyl)cyclohexanecarboxamide has a cooling equivalence to 2.0 ppm menthol (e.g. about 40X more cooling).
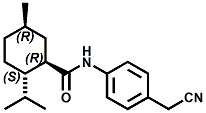
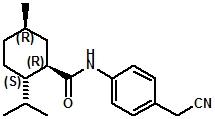
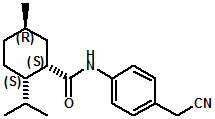
In U.S. Patent No. 7,414,152 by Galopin et.al. (Givaudan), N-(4-cyanomethylphenyl) p-menthanecarboxamide (Now FEMA 4496) was about 10X more cooling as compared to menthol at 2 ppm. This material is Givaudan's Evercool G-180. Of particular interest is the Procter & Gamble United States Patent Application 2010007608 (March 25, 2010, by K.E.Yelm, G.M Bunke & J.C. Haught, entitled Synthesis of Cyclohexane Derivatives Useful as Sensates in Consumer Products which explores the synthesis and sensory properties of various Neo-menthane carboxamides (including the Neo isomer (1S,2S,5R)-N-(4-(cyanomethyl)phenyl)-2-isopropyl-5-methylcyclohexanecarboxamide, Neo G-180).
The sensate properties indicate that the Neo G-180 isomer provides good cooling, especially in combination with G-180 in that the combination overcomes a burning sensation perceived at high levels of G-180 alone. The table below provides the evaluation system and results. 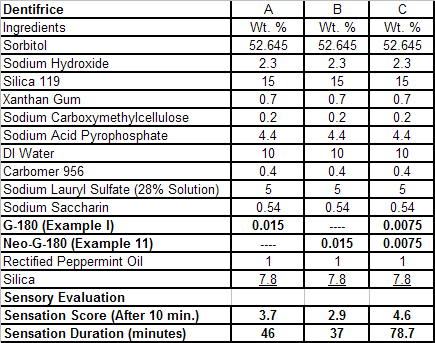
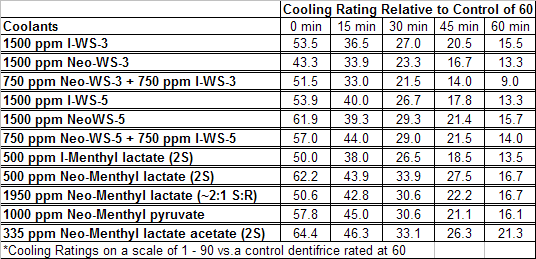 In 2007, Galopin et.al. (Givaudan) reported a series 1/7-isopropyl-4/5-methyl-bicyclo[2.2.2]oct-5-ene derivatives as having cooling properties. For example, 1-lsopropyl-4-methyl-bicyclo[2.2.21oct-5-ene-2,3-dicarbinol had a cooling intesity similar to methol at 2 ppm. See See Galopin et. al., WO2007022651 (March 1, 2007) In 2007, Galopin et.al. (Givaudan) reported that a series of compounds such as 4-methoxy-N-phenyl-N-[2-(pyridin-2-yl)ethyl]benzamide, 4-methoxy-N-phenyl-N-[2- (pyridin-2-yl)ethyl]benzenesulfonamide, 4-chloro-N-phenyl-N-[2-(pyridin-2- yl)ethyl]benzenesulfonamide and 4-cyano-N-phenyl-N-[2-(pyridin-2- yl)ethyl]benzenesulfonamid were useful as cooling agents, See Galopin et. al., WO2007048265 (May 3, 2007) In 2007, Galopin et.al. (Givaudan) reported that a series of compounds such 4-((benzhydrylamino)methyl)-2-methoxyphenol, 4-((bis(4-methoxyphenyl)-methylamino)-methyl)-2-methoxyphenol, 4-((1,2-diphenylethylamino)methyl)-2-methoxyphenol, 4- ((benzhydryloxy)methyl)-2-methoxyphenol, 4-((9H-fluoren-9-ylamino)methyl)-2- methoxyphenol or 4-((benzhydrylamino)methyl)-2-ethoxyphenol had cooling intensitie similar to or higher than menthol. See Galopin et.al., WO2007104175 (Sept. 20, 2007). In 2008, Furrer et. al. (Givaudan) reported a series of benzimidazole cooling compounds such as 1-(4-methoxyphenyl)-2-(1-methyl-1H-benzo[d]imidazol-2-yl)vinyl4-methoxybenzoate, 2-(1-isopropyl-6-methyl-1H-benzo[d]imidazol-2-yl)-1-(4-methoxyphenyl)vinyl4-methoxybenzoate and (Z)-2-(1-isoprop yl-5-methyl-1H-benzo[d]imidazol-2-y1)-1-(4-methoxy-phenyl)vinyl-4-methoxybenzoate that had cooling intensities at 0.5 ppm equivalent to 2 ppm menthol. See Furrer et. al., WO2008006236 (Jan. 17, 2008). In a collaboration between scientists at Robertet and the C.N.R. Istituto di Chimica del Riconoscimento Molecolare, different 3-alkyl-p-methan-3-ol derivatives were found to provide a strong physiological cooling effect with potential application as food and cosmetic additives. In order to investigate the influence of the chemical structure on the cooling sensation, the stereoselective syntheses of 29 different 3-alkyl-p-methan-3-ol derivatives were accomplished. 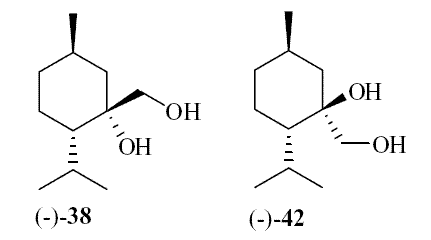 All the compounds obtained are odorless and were evaluated by taste, considering two sensations: a cooling effect and bitterness. The results of this structure–activity relationship study highlight that compounds with a (1R,4S)-configuration are the isomers with the more intense cooling effect and lower bitterness. In addition, the structure of the 3-alkyl chain affected the latter properties. Increasing the chain length over two carbon atoms does not change the cooling power, but enhances the bitterness with the additional feature that the branched isomers are considerably more bitter than the linear ones. Overall, the 3-alkyl-p-menthan-3-ol isomers with the best quality in terms of high cooling power and low bitterness are (1R,4S)-3-(hydroxymethyl)-p-menthan-3-ol diastereoisomers (-)-38 & (-)-42. See Claudio Fuganti, Daniel Joulain, Francesco Maggioni, Luciana Malpezzi, Stefano Serra, Andrea Vecchione, 3-Alkyl-p-menthan-3-ol derivatives: synthesis and evaluation of their physiological cooling activity, Tetrahedron: Asymmetry, Volume 19, Issue 20, 20 October 2008, Pages 2425-2437 and D. JOULAIN; C. FUGANTI; S. SERRA; A.VECCHIONE, P-MENTHAN-(3) OL ALKYLATED DERIVATIVES AND USE THEREOF AS REFRESHING AGENTS, WO2005121058 (A1), Dec. 22, 2005. Another recent patent application ( United States Patent Application 20080319055, Dec. 25, 2008) by Lucienne Cole et al. entitled Cooling Compounds discloses a series of new amides with cooling properties. For example, N-(1-methyl-1-isopropylbutyl)benzamide is reported to be about 20X cooler than menthol. 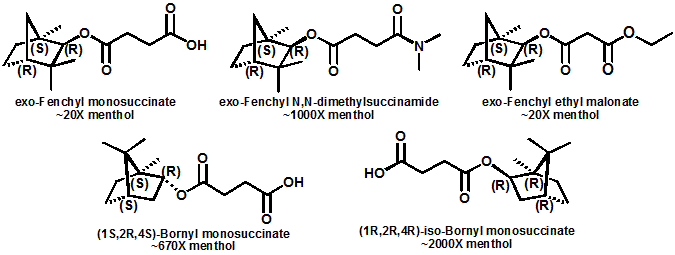 In United States Patent Application 20080311232 (Dec. 18, 2008) and United States Patent 7959958 (June 14, 2011) entitled "Cooling Compounds", Furrer et al. disclose a series of derivatives of fenchyl, D-bornyl, L-bornyl, exo-norbornyl, 2-methylisobornyl, 2-ethylfenchyl, 2-methylbornyl, cis-pinan-2-yl, verbanyl and isobornyl with cooling intensities up to 2000X cooler than menthol (i.e., the isoborneol derivative, 4-oxo-4-((lR,2R,4R)-1,7,7-trimethylbicyclo[2.2. l]heptan-2-yloxy)butanoic acid). In United States Patent Application 20090054520 (Feb. 26, 2009), Surburg et al. at Symrise disclose a series of menthyl oxamate derivatives useful as cooling agents. In United States Patent Application 20080175800 (July 24, 2008), Schoening & Wiedwald at Symrise describe the use of specific menthyl 3-oxocarboxylic acid esters (e.g. L-menthyl 3-oxobutyrate and L-menthyl 3-oxopentanoate) as cooling agents. In United States Patent Application 20080096969 (April 24, 2008) entitled N alpha-(Menthanecarbonyl)amino acid amides and use thereof as physiological cooling active ingredients, Jacob Ley discloses a series of cooling agents related to WS-5. 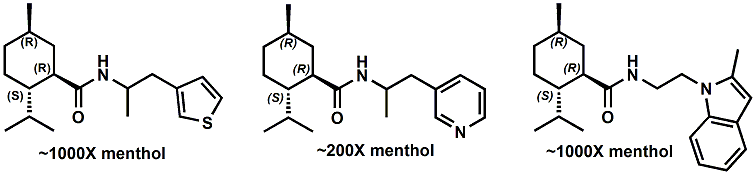 In WIPO Patent Application WO/2010/128026 Furrer discloses a series of new p-menthane carboxamide and WS-23 analogs as cooling agents. Three particularly potent cooling agents are shown above. 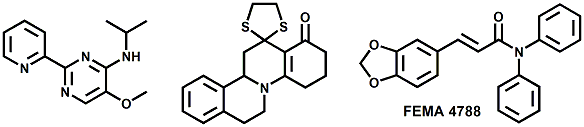 In WIPO Patent Application WO/2011/061330 (May 26, 2011) Horst Surburg et al. at Symrise, disclose a number of structurally new cooling agents, some of which have long lasting cooling duration and are cooler than WS-3. Three of these are depicted above. A number of these materials are also disclosed in United States Patent Application 20110145970 (June 23, 2011) by Thomas Subkowski et al. based on the screening of novel modulators against the cold menthol receptor TRPM8. FEMA 4788, "3,4-methylenedioxycinnamic acid, N,N-diphenylamide" was perceived to be almost twice as cooling as WS-3 (3-menthane carboxylic acid-N-ethylamide) and much longer lasting. Several interesting papers on cooling agents have recently appeared - a) Stefan M. Furrer, Jay P. Slack, Scott T. McCluskey, Ioana M. Ungureanu, Andrew T. Daniher, Guillaume Blancher, Karen Bell, Pablo Krawec, Lucienne Cole and Kim Gray, New Developments in the Chemistry of Cooling Compounds, Chemosensory Perception, Volume 1, Number 2 / June, 2008, pp 119-126; b) John C. Leffingwell, Cooling Ingredients and Their Mechanism of Action, in Handbook of Cosmetic Science and Technology, Third edition, André O. Barel, Marc Paye, and Howard I. Maibach, Editors, Pub: Informa Healthcare: New York. Chapter 65, pp. 661-675 (2009). See also the Society of Flavor Chemists presentation "New Cooling Compounds". 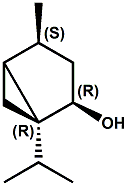
(-)-(1R,2R,4S)-dihydroumbellulol Christian Starkenmann, Isabelle Cayeux, Robert Brauchli, and Fabienne Mayenzet, Hemisynthesis of Dihydroumbellulols from Umbellulone: New Cooling Compounds, J. Agric. Food Chem., 2011, 59 (2), pp 677–683 have reported that that (1R,2R,4S)-dihydroumbellulol has a pleasant, trigeminal cooling effect, about 2-3 times less cooling than (-)-menthol, with a weak odor slightly reminiscent of eucalyptol. See also WIPO Patent Application WO/2011/138696 (Nov. 10, 2011) assigned to Firmenich. 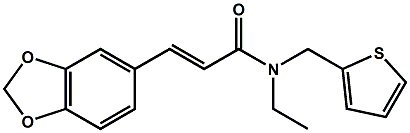
Senomyx candidate In WIPO Patent Application WO/2012/061698 by C. Priest et al. entitled Compounds useful as modulators of TRPM8 (May 10, 2012) assigned to Senomyx, an extensive list of potential new cooling compounds are described. Like most Senomyx patent applications and patents, it is a task to find the jewels in the haystack. Nevertheless, compounds like the one shown above appear to have strong cooling potency based on EC50 (uM) values. 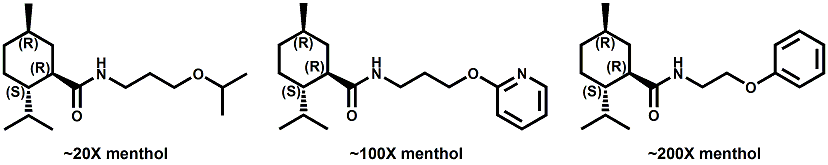 In United States Patent No. 8377422 (Feb.19,2013) by Furrer et al., assigned to Givaudan, a series of p-menthane alkyloxy amides (shown above) were patented that have 20-200X cooling as compared to menthol at 2 ppm. 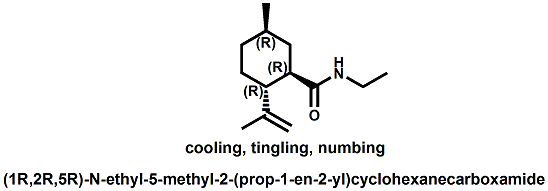 In WIPO Patent Application WO/2013/033501 (March 7, 2013) by Lombardo et al. entitled NOVEL SUBSTITUTED CYCLOHEXANE COMPOUNDS, an extensive series of Isopulegol derived carboxamide derivatives are disclosed that have a wide rang of organoleptic / sensate properties. One of the most interesting compounds was the WS-3 analog (1R,2R,5R)-N-ethyl-5-methyl-2-(prop-1-en-2-yl)cyclohexanecarboxamide that was "cooling, tingling, numbing" and enhances the warming effect of pungent compounds much better than WS-3. This material was assigned FEMA No. 4808 in GRAS list No. 27. In United States Patent Application 20130216486 (Aug. 22, 2013) by Yelm et al., assigned to Procter & Gamble, entitled SYNTHESIS OF CYCLOHEXANE DERIVATIVES USEFUL AS SENSATES IN CONSUMER PRODUCTS, a series of Neomenthyl esters and ethers were disclosed that had cooling properties as good as, or better, than the coresponding menthyl isomers. 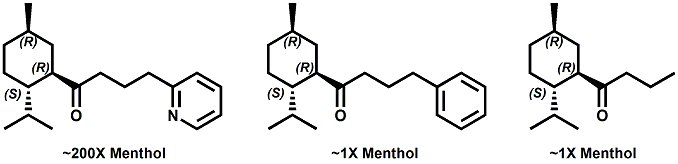 In United States Patent 7935848 (May 5, 2011) by Furrer et al., assigned to Givaudan, entitled Butone derivatives useful as cooling agents, a series of menthyl ketones were disclosed with cooling properties as cool or cooler than menthol. 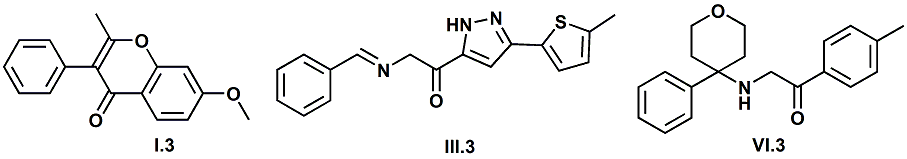 In Krohn, Michael, and Holger Zinke. "Small molecule modulators of the cold and menthol receptor TRPM8." U.S. Patent Application 14/003,762, filed March 8, 2012. assigned to B.R.A.I.N. Biotechnology Research and Information Network AG, the compounds I.3, III.3 and Vi.3 (shown above) were found to have a TRPM8 cooling efficacy similar to menthol.
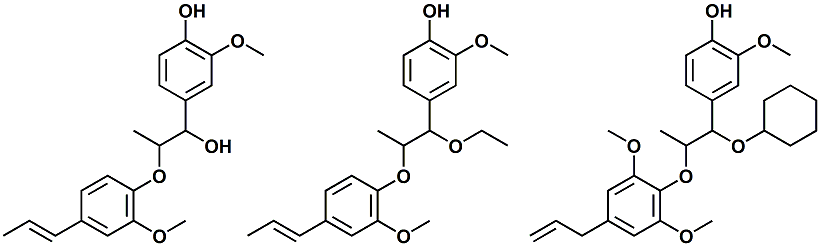 In WIPO Patent Application WO/2013/080830 (06/06/2013) entitled Cooling agent and TRPM8 activator ,by Tomohiro Shirai et al., assigned to Kao Corporation, a series of lignin type derivatities (as shown above) are disclosed as TRMP8 activators and cooling agents. See also, Tomohiro Shirai et al., Processed nutmeg product and method for producing same, WIPO Patent Application WO/2014/010657, Published: 01/16/2014.
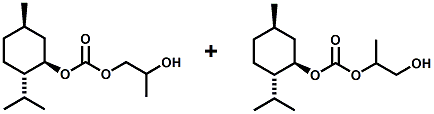
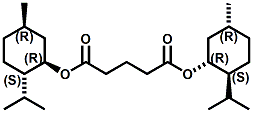
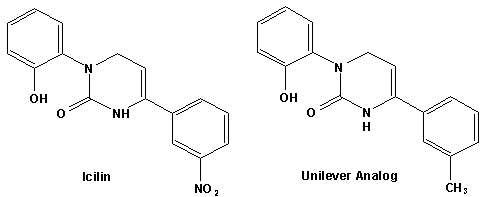
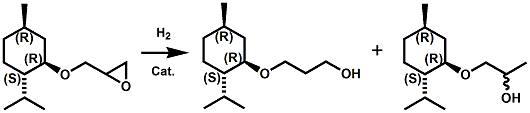 In European Patent Application EP2772477 (09/03/2014) by Shigeru Tanaka and Kenya Ishida of Takasago disclosed that a mixture of 3-menthoxy-1-propanol and 1-menthoxy-2-propanol (shown above) imparts a refreshing cooling sensation and improves the refreshing cooling sensation when admixed at a 5% level with menthol. In United States Patent Application 20140219930 (08/07/2014) by John V. Thumpalasseril et al. (assigned to International Flavors & Fragrances) entitled "Cooling Enhancing Compositions" it is disclosed that the cooling effect of physiological cooling agents, such as menthol, WS-3, WS-5, menthyl glutarate etc., ca be enhanced by using a cooling enhancing composition comprising eriodictyol, homoeriodictyol, hespertin and mixtures thereof. In United States Patent Application 20150139918 (05/21/2015) by Rita Lages et al. (assigned to Symrise) entitled "Mixtures having improved cooling effect", it is disclosed that certain phenylalkenals (e.g. 2-Phenyl-but-2-enal or 2-Phenyl-pent-2-enal) synergistically enhance the cooling effect of physiological cooling agents, and even a reduction of stinging, burning or bitterness may be achieved. ------------------------------------------------------------------ Icilin also known as AG-3-5, chemical name 1-[2-hydroxyphenyl]-4-[2-nitrophenyl- ]-1,2,3,6-tetrahydropyrimidine-2-one) was discovered in 1983 to produced sensations of coldness when in contact with mucous membranes (nostrils, lips and eyelids) of the researchers, and also when ingested (see Wei et al, J. Pharm. Pharmacol. 1983, 35:110-112). It is recognized that Icilin is a considerably more potent coolant than menthol. It is a super agonist that is 2.5-fold more efficacious and nearly 200-fold more potent than the reference cold thermosensory agonist, l-menthol. However, David A. Andersson and coworkers at Novartis have shown that the that the activation of TRPM8 cold receptor by icilin and cold, but not menthol, is modulated by intracellular pH in the physiological range. Their data suggests that activation by icilin and cold involve a different mechanism to activation by menthol. See David A. Andersson, Henry W. N. Chase, and Stuart Bevan, TRPM8 Activation by Menthol, Icilin, and Cold Is Differentially Modulated by Intracellular pH, Journal of Neuroscience, June 9, 2004, 24(23):5364-5369.
In April of 2004, Unilever Home & Personal Care USA, A Division of Conopco, Inc., disclosed a series of Icilin analogs that possess cooling potency similar to menthol (using cellular fluorescence)30. A number of patents using these materials (often in combination) in flavoring, perfume, cosmetic and oral care products have been issued. Some of these are listed below22- . Cooling Compounds as Insect Repellents Recently, it has been discovered that a number of cooling agents also have potent Insect repelling activity. In particular, Gautschi & Blondeau of Givaudan (United States Patent Application 20040028714, February 12, 2004) have discovered that WS-3 (N-Ethyl-p-menthane-3-carboxamide) and related N-substituted p-menthane carboxamides have insect repelling activity equal to or exceeding that of DEET (Diethyl-m-toluamide).The following example compares the repellency of WS-3 vs. DEET.
Similarly, the p-Menthane-3,8-diols are known insect repellents (N. Hiroyuki, et. all, Japanese Patent No. 60199804, Sept 10, 1985; Sikinami, et al., United States Patent 5,017,377, May 21, 1991) and have recently been reviewed (Barnard, D.R. – Repellents and toxicants for personal protection – Position paper. Geneva, World Health Organization, 2000) Quwenling, a popular Eucalyptus-based repellent product, contains a mixture of p-menthane-3,8-diol (PMD), isopulegone and citronellol. Quwenling has largely replaced dimethyl phthalate as the insect repellent of choice in China. Citrepel®, a natural PMD product is produced by Chemian Technology Ltd. Barnard, et. al., compared the mean percent repellency for Merck's IR3535® (a component in Avon's SKIN-SO-SOFT Bug Guard PLUS IR3535® Insect Repellent ), Bayer's KBR3023 (Bayrepel®), para-Menthane-3,8-diol (PMD), and DEET against black salt marsh mosquitoes. In J Med Entomol. 2002 Nov;39(6):895-9.
Questice® (menthyl pyrrolidone carboxylate) has also been patented as an insect repellent (Watkins, et al., United States Patent 6,451,844, September 17, 2002) and Kalbe and Nentwig in German Patent No. 19840321 describe the use of menthyl lactate or menthol glycerol acetal for repelling mites and other insects. In addition Watkins, et al., compared the repellent activity of Questice® vs. DEET and Menthol against mesquitoes.
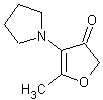
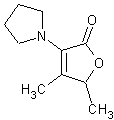
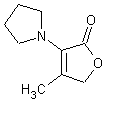
In November 2001, T. Hoffmann and co-workers in Germany reported23 a compound that occurs naturally in Malt with much greater cooling power than menthol. They say the most active compound, 4-methyl-3-(1-pyrrolidinyl)-2[5H]-furanone, which is from a family called cyclic alpha-keto enamines, is 35 times more powerful in the mouth, and 512 times more powerful on the skin, than menthol, the active ingredient in mint (based on threshold value ratios). The cooling effect also lasts twice as long. The three most active compounds synthesized are shown below.
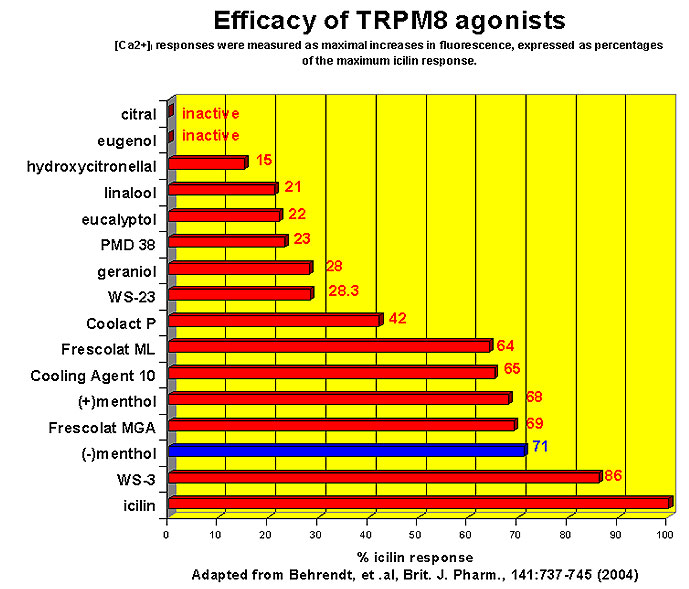
This research received considerable press coverage [ Today's Chemist (February 2002), Science News (Dec. 15, 2001), AAAS Eureka Alert (November 6, 2001), Irish Examiner (December 13, 2001), ScienceBase.com - Elemental Discoveries (December 2001), Deutsche Forschungsanstalt für Lebensmittelchemie Annual Report Summaries, 2001, The Hindu (Nov 22, 2001), ABC-Australia (Great Moments in Science), etc.], several of which quote the researchers as saying: ''We've found the world's most powerful natural cooling agents without a mint odour.'' However, at the December 4, 2003 meeting of the Society of Flavor Chemists meeting, industry sources revealed that in "actual practice" the cooling power of 4-methyl-3-(1-pyrrolidinyl)-2[5H]-furanone was very much lower than expected and this material was of little interest. 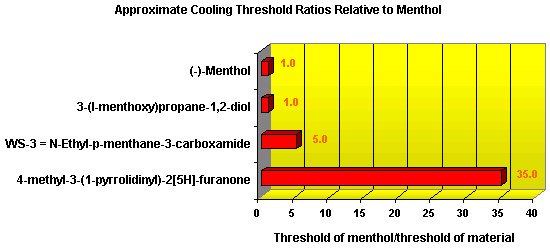 Thus, it appears that the use of reported cooling threshold levels to estimate "actual cooling strength" can give erroneous results. At this same meeting, Mark Erman of Millennium Specialty Chemicals provided extensive information on the relative cooling strength of a number of cooling compounds. With permission, we have posted Dr. Erman's presentation on our site. This cooling strength information is provided in chart form below. Structures of the various WS compounds may be found in Dr. Erman's presentation. 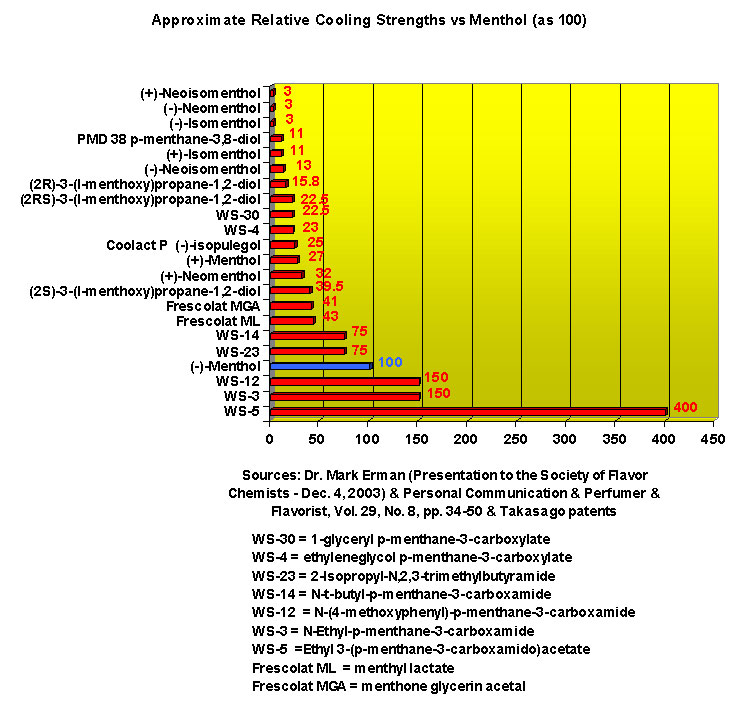 Physiology of Cooling from materials such as menthol The action of menthol and similar coolant compounds on "thermoreceptors" provides the "cool" sensation via cold receptors. In the case of menthol and certain other coolant compounds one can also get a "hot" or stinging "pain" sensation. Menthol can act at high concentrations in much the same way as capsaicin to produce a hot sensation, but in this case, it stimulates the fibers that register both cold temperatures as well as those that respond to warmth. Recently (2001), Gordon Reid & Maria-Luiza Flonta at the University of Bucharest have discovered an inward ionic current that is activated by moderate cooling in a small number of rat sensory neurons. This current has features that are found in intact cold receptors, including sensitization by menthol, adaptation upon sustained cooling, and modulation by calcium, and is likely to be important in cold sensing. Early models indicated that menthol stimulates cold receptors by blocking voltage-dependent Ca2+ channels, leading to a reduction in intracellular Ca2+ and inhibition of Ca2+-dependent K+ channels5. However, Reid and others have since discovered that menthol stimulates entry of Ca2+ and increases intracellular Ca2+ concentration in cold-sensitive neurons; thus stimulation of cold receptors by menthol can be explained more simply by sensitization of the cold-induced inward Ca2+ current. In the March 7, 2002 issue of Nature, McKemy, Neuhausser & Julius20 have characterized and cloned a menthol receptor from trigeminal sensory neurons that is also activated by thermal stimuli in the cool to cold range. This cold- and menthol-sensitive receptor, CMR1, is a member of the TRP family of excitatory ion channels, and they propose that it functions as a transducer of cold stimuli in the somatosensory system. These findings, together with their previous identification of the heat-sensitive channels VR1 and VRL-1, demonstrate that TRP channels detect temperatures over a wide range and are the principal sensors of thermal stimuli in the mammalian peripheral nervous system. In Nature 416, 52–58 (7 March 2002). In the same issue of Nature, Charles Zucker19 explains how the discovery of a cold-sensitive ion channel will help dissect how the nervous system encodes and decodes the temperature spectrum. In Nature 416, 27–28 (7 March 2002) Similarly, Andrea Peier et. al. described the cloning and characterization of TRPM8, a receptor activated by cold temperatures and by the cooling agent, menthol. In Cell,108(5):705-15 ( Mar 8, 2002)22 Also in March 2002, Viana & co-workers21 results suggest that cold sensitivity is not associated to a specific transduction molecule but instead results from a favorable blend of ionic channels expressed in a small subset of sensory neurons. In Nat Neurosci 2002 Mar;5(3):254-60 In 2003, Patapoutian, et. al., reviewed the statue of the mechanisms of temperature sensations. In Nature Reviews Neuroscience 4, 529-539 (2003) In February 2004, H-J Behrendt, et. al., published a study on the effects of 70 odorants and menthol-related substances on recombinant cold-menthol receptor TRPM8 (mTRPM8), expressed in HEK293 cells. These were examined using a fluorometric imaging plate reader (FLIPR®) assay. In all, 10 substances (linalool, geraniol, hydroxycitronellal, WS-3, WS-23, FrescolatMGA, FrescolatML, PMD38, CoolactP and Cooling Agent 10) were found to be agonists. A summary of the potencies and efficacies are shown below (as adapted from their data). With a few exceptions, the data is in good agreement with the sensory data shown in the graphs above. This work gives a new approach for screening cooling compounds. In British Journal of Pharmacology 141:737-745, 2004. However, David A. Andersson and coworkers at Novartis have shown that the that the activation of TRPM8 cold receptor by icilin and cold, but not menthol, is modulated by intracellular pH in the physiological range. Their data suggests that activation by icilin and cold involve a different mechanism to activation by menthol. See David A. Andersson, Henry W. N. Chase, and Stuart Bevan, TRPM8 Activation by Menthol, Icilin, and Cold Is Differentially Modulated by Intracellular pH, Journal of Neuroscience, June 9, 2004, 24(23):5364-5369. 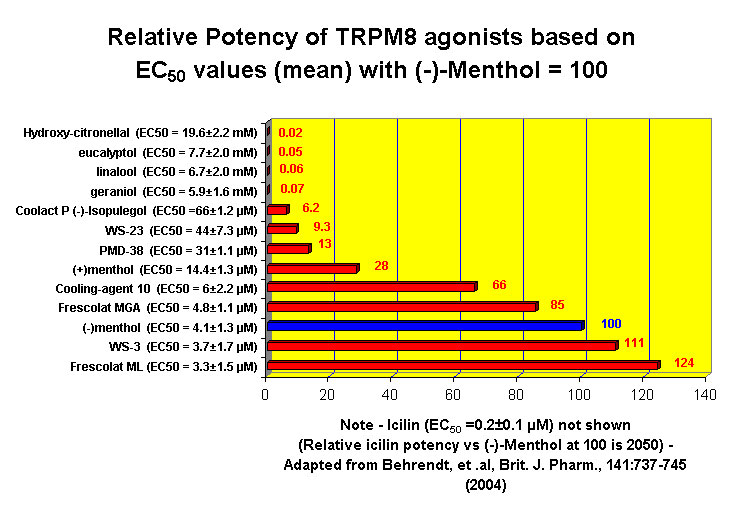
In April 2005, McKemy published a review entitled "How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation" that provides current insight. In Molecular Pain 2005, 1:16 doi:10.1186/1744-8069-1-16 For those interested see Physiology of Cooling references below. 1. Watson, H. R., "Flavor Characteristics Of Synthetic Cooling Compounds" in Flavor: It's Chemical, Behavioral & Commercial Aspects; C. M. Apt, Ed., Westview Press, Boulder, 1977, pp. 31-50. 2. Watson, H. R., R. Hems, D.G. Roswell & D. J. Spring, New Compounds with the Menthol Cooling Effect, J. Soc. Cosmet. Chem., 29, 185-200 (1978). 3. Roswell, David G.; Spring, David J.; Hems, Roger, Acyclic carboxamides having a physiological cooling effect, United States Patent 4,296,255, October 20, 1981, Assigned to Wilkinson Sword Limited. 4. Rowsell, David G.; Gascoyne, John M.; Hems, Roger, Cyclic carboxamides having a physiological cooling effect , United States Patent 4,296,093, October 20, 1981, Assigned to Wilkinson Sword Limited. 5. Roswell, David G.; Spring, David J.; Hems, Roger, Acyclic carboxamides having a physiological cooling effect, United States Patent 4,230,688, October 28, 1980, Assigned to Wilkinson Sword Limited. 6. Watson, Hugh R.; Rowsell, David G.; Spring, David J., N-substituted paramenthane carboxamides, United States Patent 4,226,988, October 7, 1980, Assigned to Wilkinson Sword Limited. 7. Watson, Hugh R.; Rowsell, David G.; Spring, David J., N-substituted paramenthane carboxamides, United States Patent 4,193,936, March 18, 1980, Assigned to Wilkinson Sword Limited. 8. Watson, Hugh R.; Rowsell, David G.; Spring, David J., N-substituted paramenthane carboxamides, United States Patent 4,178,459, December 11, 1979, Assigned to Wilkinson Sword Limited. 9. Roswell, David G.; Spring, David J.; Hems, Roger, Acyclic carboxamides having a physiological cooling effect, United States Patent 4,153,679, May 8, 1979, Assigned to Wilkinson Sword Limited. 10. Watson, Hugh R.; Rowsell, David G.; Spring, David J., N-substituted paramenthane carboxamides, United States Patent 4,150,052, April 17, 1979, Assigned to Wilkinson Sword Limited. 11. Rowsell, David G.; Spring, David J., Phosphine oxides having a physiological cooling effect, United States Patent 4,070,496, January 24, 1978, Assigned to Wilkinson Sword Limited. 12. Rowsell, David G.; Hems, Roger, Compounds having a physiological cooling effect and compositions containing them, United States Patent 4,070,449, January 24, 1978, Assigned to Wilkinson Sword Limited. 13. Watson, Hugh R.; Rowsell, David G.; Spring, David J., Tobacco and tobacco-containing manufactures containing an ingredient having physiological cooling activity, United States Patent 4,060,091, November 29, 1977, Assigned to Wilkinson Sword Limited. 14. Watson, Hugh R.; Rowsell, David G.; Browning, John H. D., Tobacco and tobacco-containing manufactures containing an ingredient having physiological cooling activity, United States Patent 4,059,118, November 22, 1977, Assigned to Wilkinson Sword Limited. 15. Rowsell, David G.; Hems, Roger, Compounds having a physiological cooling effect and compositions containing them, United States Patent 4,034,109, July 5, 1977, Assigned to Wilkinson Sword Limited. 16. Watson, Hugh R.; Rowsell, David G.; Browning, John H. D., Substituted p-menthanes, United States Patent 4,033,994, July 5, 1977, Assigned to Wilkinson Sword Limited. 17. Rowsell, David G.; Spring, David J., Cyclic sulphoxides and sulphones having a physiological cooling action on the human body, United States Patent 4,032,661, June 28, 1977, Assigned to Wilkinson Sword Limited. 18. Rowsell, David G.; Spring, David J., Cyclic sulphonamides and sulphinamides having a physiological cooling effect, United States Patent 4,020,153, April 26, 1977, Assigned to Wilkinson Sword Limited. 19. Grub, Helmut; Pelzer, Ralf; Hopp, Rudolf; Emberger, Roland; Bertram; Heinz-Jurgen, Compositions which have a physiological cooling effect, and active compounds suitable for these compositions, United States Patent 5,266,592, November 30, 1993, Assigned to Haarmann & Reimer GmbH . 20. Amano, Akira; Moroe, Michio; Yoshida, Toshio, 3-Levo-Menthoxypropane-1,2-diol, United States Patent 4,459,425, July 10, 1984. Assigned to Takasago Perfumery Co., Ltd. 21. Yamamoto, Takeshi; Method for purifying (-)-N-isopulegol and citrus perfume composition containing (-)-N-isopulegol obtained by the method, United States Patent 5,773,410, June 30, 1998. Assigned to Takasago International Corporation. 22. Suares; Alan Joseph; Znaiden; Alexander Paul; Feliciano; Donald Carl; Carrabotta; Michele, Cosmetic compositions with sensate mixtures based on isopulegol, United States Patent 6,267,974, July 31, 2001. Assigned to Unilever Home & Personal Care USA, Division of Conopco, Inc. 23. Ottinger, H., T. Soldo & T. Hoffmann, Systematic Studies on Structure and Physiological Activity of Cyclic alpha-Keto Enamines, A Novel Class of "Cooling Compounds", J. Agric. Food Chem., 49, 5383-5390 (2001). See also Hofmann, Thomas; Ottinger, Harald; Frank, Oliver; Soldo, Tomislav; Cerny, Christoph; Robert, Fabien; Blank; Imre; Method of using alpha-keto enamine derivatives as ingredients and products incorporating same, United States Patent 6,592,884, July 15, 2003 . 24. Kenmochi; Hiroyuki; Akiyama; Teruyoshi; Yuasa; Yoshifumi; Kobayashi; Toyohiko; Tachikawa; Akio, Method for producing para-menthane-3,8-diol, United States Patent 5,959,161, September 28, 1999. Assigned to Takasago International Corporation. 25. Shiroyama; Kenichiro; Sawano; Kiyohito; Ohta; Hideaki , Cool feeling composition, United States Patent 6,328,982, December 11, 2001. Assigned to Takasago International Corporation. 26. Frerot; Eric & Van Beem; Nicole, Compounds derived from menthol and use as refreshing agent, United States Patent 6,359,168, March 19, 2002 . Assigned to Firmenich SA. 27. Velazco; Maria Ines; Wuensche; Laurent; Deladoey; Patrice, Use of cubebol as a flavoring ingredient, United States Patent 6,214,788, April 10, 2001. Assigned to Firmenich SA. 28. Amano; Akira, Tokoro; Kazuhiko, (2S)-3-[(1R, 2S, 5R)-[5-methyl-2-(1-methylethyl)-cyclohexyl]oxy]-1, 2-propanediol, process for producing the same, and compositions containing the same, United States Patent 5,608,119, March 4, 1997. Assigned to Takasago International Corporation. 29. TAKAZAWA OSAMU; WATANABE HIROYUKI; ISO MASAKI , p-MENTHANE DERIVATIVE AND COLD SENSING AGENT CONTAINING THE SAME, Japanese Patent No. 2004059474, February 26, 2004. Assigned to T. Hasegawa Co., Ltd. 30. Foster, Alison Jane; Van Der Logt, Cornelius Paul Erik; Tareilus, Erwin Werner; Novel compounds and their uses, United States Patent Application 20040067970, April 8, 2004. Assigned to Unilever. 31. GALOPIN, CHRISTOPHE; KRAWEC, PABLO VICTOR; SLACK, JAY PATRICK; TIGANI, LORI, N-SUBSTITUTED P-MENTHANE CARBOSAMIDED, WO Patent No. 2005049553, June 2, 2005. Assigned to Givaudan. 32. Dewis; Mark L.; Huber; Michelle E.; Cossette; Michael V., Menthyl half acid ester derivatives, processes for preparing same, and uses thereof for their cooling/refreshing effect in consumable materials, United States Patent 6,884,906, April 26, 2005. Assigne to International Flavors & Fragrances Inc.
Physiology of Cooling References: 1.Reid G, Flonta ML., Cold current in thermoreceptive neurons, Nature, Oct 4; 413, 480 (2001) 2. Hensel, H. Thermoreception and Temperature Regulation (Academic, London, 1981). 3. Reid, G. & Flonta, M.-L. Neurosci. Lett. 297, 171–174 (2001). 4. Suto, K. & Gotoh, H. Neuroscience 92, 1131–1135 (1999). 5. Reid, G., Amuzescu, B., Zech, E. & Flonta, M.-L. J. Neurosci. Methods 111, 1–8 (2001). 6. Gee, M. D., Lynn, B., Basile, S., Pierau, F. K. & Cotsell, B. Neuroscience 90, 509–518 (1999). 7. Leem, J. W., Willis, W. D. & Chung, J. M. J. Neurophysiol. 69, 1684–1699 (1993). 8. Kenshalo, D. R. & Duclaux, R. J. Neurophysiol. 40, 319–332 (1977). 9. Hensel, H. & Zotterman, Y. Acta Physiol. Scand. 24, 27–34 (1951). 10. Schäfer, K., Braun, H. & Isenberg, C. J. Effect of menthol on cold receptor activity. Analysis of receptor processes, Gen. Physiol. 88, 757–776 (1986). 11. Hensel, H. & Schäfer, K. Pflügers Arch. 352, 87–90 (1974). 12.Okazawa, M., Terauchi, T., Shiraki, T., Matsumura, K. & Kobayashi, S. NeuroReport 11, 2151–2155 (2000). 13. Pierau, F., Torrey, P. & Carpenter, D. Brain Res. 73, 156–160 (1974). 14. Eccles R., Menthol and related cooling compounds, J Pharm Pharmacol Aug; 46(8):618-30 (1994) 15. Wright CE, Bowen WP, Grattan TJ, Morice AH., Identification of the L-menthol binding site in guinea-pig lung membranes, Br J Pharmacol, Feb;123(3):481-6 (1998) 16. Schafer K., A quantitative study of the dependence of feline cold receptor activity on the calcium concentration, Pflugers Arch 1987 Jun;409(1-2):208-13 17. Schafer K, Braun HA, Rempe L., Classification of a calcium conductance in cold receptors, Prog Brain Res 1988;74:29-36 18. Eccles R, Role of cold receptors and menthol in thirst, the drive to breathe and arousal, Appetite 2000 Feb;34(1):29-35 19. Zuker, C.S., Neurobiology: A cool ion channel., Nature 2002 Mar 7;416(6876):27-8. 20. McKemy DD, Neuhausser WM, Julius D., Identification of a cold receptor reveals a general role for TRP channels in thermosensation.. Nature 2002 Mar 7;416(6876):52-8 21. Viana, Félix, Elvira de la Peña and Carlos Belmonte, Specificity of cold thermotransduction is determined by differential ionic channel expression, Nat Neurosci 2002 Mar;5(3):254-60; Comment in: Nat Neurosci. 2002 Mar;5(3):189-90. 22. Andrea M. Peier, Aziz Moqrich, Anne C. Hergarden, Alison J. Reeve, David A. Andersson, Gina M. Story, Taryn J. Earley, Ilaria Dragoni, Peter McIntyre, Stuart Bevan, and Ardem Patapoutian; A TRP Channel that Senses Cold Stimuli and Menthol, Cell. 2002 Mar 8;108(5):705-15 23. Okazawa M, Terauchi T, Shiraki T, Matsumura K, Kobayashi S., l-Menthol-induced [Ca2+]i increase and impulses in cultured sensory neurons., Neuroreport 2000 Jul 14;11(10):2151-5. 24. H-J Behrendt, T Germann, C Gillen, H Hatt and R Jostock, Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay, Br J Pharmacol. 2004 Feb;141(4):737-45. Epub 2004 Feb 02
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY