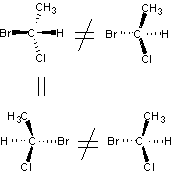by John C. Leffingwell, Ph.D.
Photo by permission of M. Roudintska - Art & Parfum
|
|
Ionones, Irones, Damascones & Structurally Related Odorants |
|
|
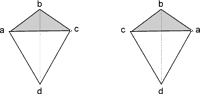
As of April 6, 2010 You must have Java installed to view the molecular visualization. Chirality & Odour Perception It had been recognized by chemists in the flavor & fragrance industry since the beginning of the 20th century that certain enantiomeric chemicals, such as menthol and carvone had different & differentiating organoleptic properties. Perhaps the first rigorously definitive works were by Julius von Braun culminating in the synthesis and odor evaluation of the enantiomers of 3,5-dimethylcyclohexanone and the 3,5-dimethylcyclohexanols (see Geruch und molekulare Asymmetrie, IV. Mitteilung: Die drei 1.3-Dimethylcyclohexanone-5 und die vier 1.3-Dimethyl-cyclohexanole-5, Berichte der deutschen chemischen Gesellschaft (A and B Series), Volume 60, Issue 11, Date: 7. Dezember 1927, Pages: 2438-2446). By the 1960's, a number of processes had been developed for the synthesis of the desired (-)-menthol from optically active terpenoids and both (-)-carvone and (+)-carvone were being manufactured from (+)- and (-)-limonene respectively by Norda (--> then Quest International --> now Givaudan). However, the premise that optical enantiomers could have different odours was not generally accepted by various academics (based partly on an erroneous theory of olfaction proposed by Wright) until the mid-1970's to 1980's's. Admittedly, prior to gas chromatography and other measurments of purification techniques, purity of the enantiomers used for the odour evaluations was always a question. In addition, a high enantiomeric excess for the chemical is nearly always required for organoleptic evaluations. A number of reviews on this subject have been written, notably those by Boelens1, Ohloff 2 and Pickenhagen 3. However, many new chemicals and their enantiomeric odour descriptions have been reported since these prior reviews were written. In this article, we have compiled a list of chemicals for which enantiomeric odour discrimination is known. Such descrimination is defined as: differing odour descriptors or/and odor strength as determined by threshold measurements. We greatly appreciate information provided to us by Philip Kraft of Givaudan who along with his associates (George Frater, Riccardo Cadalbert, Caroline Denis, Jerzy Bajgrowicz, Markus Gautschi, Roman Kaiser, Urs Muller & Walter Eichenberger) have recently published a number of articles on this subject.12-19 These references are cited below. In addition, in January 2003, Elisabetta Brenna, Claudio Fuganti and Stefano Serra published an excellent review entitled "Enantioselective perception of chiral odorants" (Tetrahedron: Asymmetry, 14 (1), 1–42, 2003) Because of the very recent defining of the human odour receptors by Lancet, et. al, and Zozulya, et. al, we thought that making available in concise form, with accurate molecular structures and molecular models (that are acknowledged olfactorily as 3-D dependant) would be a benefit to those working on the definititive concepts of olfaction. Introduction to Chirality: The concept of "chirality" has been known in chemistry since the 1870's although it would be nearly a hundred years before chemists began using this term. In fact, in the first edition of Eliel's "Stereochemistry of Carbon Compounds" in 19624, the word chiral is not mentioned, although it would be prominant in later editions5. In extremely simple terms, chirality is "handedness," - that is, the existence of left/right opposition. For example, your left hand and right hand are mirror images and therefor "chiral". The term Chiral is derived from the greek name kheir meaning "hand" and apparently was coined by Lord Kelvin in 1904, in his Baltimore Lectures on Molecular Dynamics and the Wave Theory of Light in which he stated ..."I call any geometrical figure, or group of points, chiral, and say it has chirality, if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself." For a simple video depiction see "Mirror Molecule: Carvone" at NBC Learn. While the concepts of "asymmetry" were developed by J.H. vant Hoff 6 and J.A. Le Bel7 in 1874 following the resolution by Louis Pastuer of a mixture of tartaric acid salt isomers during the period 1848-1853, in which he picked out the differing crystal types by hand - doing so on the basis of the differing physical appearance of the salt crystals8. Pastuer recognized that two of the isomers polarized light differently (one to the left and the other to the right) and that this must be due to an asymmetric grouping of atoms in the optically active molecules. Following Kekule's recognition in 1858 that carbon has a valence of 49, vant' Hoff and Le Bel independently recognized that when four different groups are attached to a carbon atom, arrayed at the corners of a tetrahedron, then the arrangements can be in two different forms, as depicted schematically to the right. As the number of carbons with assymetry (chirality) increase in a molecule the number of possible optical isomers (enantiomers) also increases. With one asymmetric carbon, 2 isomers...with two asymmetric carbons, 4 isomers, with three asymmetric carbons, 8 isomers...that is, the number of isomers is 2n, where n = number of asymmetric atoms. In the early days, chemists often assigned trivial names to differentiate isomers, and enantiomers generally were specified by d- = dextrorotary and l- = leavorotary based on which direction the molecles polarized light. But Cahn, Ingold and Prelog10 devised a system based on assigning sequence rules based on decreasing atomic number (and respective rate of substitution for atoms of the same atomic number) for projection formulas that allows the absolute configuration assignments of R (for rectus, Latin for right) and S (for sinister, Latin for left). Tutorials on the Cahn-Ingold-Prelog R/S notation are available on the internet.11 These rules are incorporated in the chirality monitor of Accelrys DS Viewer and Discovery Studio Visualizer (which is free). Very occasionally, DS ViewerPro & Discovery Studio Visualizer provides incorrect assignments (for example, with the enantiomers of gamma-dihydroionone, gamma-damascone, gamma-ionone & methyl-gamma-cyclogeranate, etc.). However, Cambridegsoft's ChemDraw Ultra appears to provide 100% correct C-I-P R,S assignments. Thus, even without knowing the sequence rules, chemist's today can rapidly establish the R/S configuration at each asymmetric atom for a given molecular structure in just a few minutes. The following tables link to molecular visualizations of over 1,400 enantiomers (>700 enantiomeric pairs) with odour descriptors and references. Note: Odour thresholds are from evaluations in water unless otherwise specified. Technical Notes: The 2D-molecular representations were built with the ChemDraw program. The 3-D mol models were prepared first by 3-D optimization Chem3D Ultra which were then verified in Accelrys DS ViewerPro or Discovery Studio Visualizer. As this works poorly (conformationally) for certain terpenoids and can be totally inaccurate in absolute configuration for some bicyclics and sesquiterpenoids, many of the more complicated molecules were constructed and energy minimized in Cambridegsoft's Chem3D or AccuModel and then verified. References: 1. Mans H. Boelens, Harrie Boelens & Leo J. van Gemert, Perfumer & Flavorist, Vol. 18, No. 6, 1-15, (1993) 2. G. Ohloff, Scent and Fragrances, Springer-Verlag (1994) 3. W. Pickenhagen, Enantioselectivity in Odor Perception, in Flavor Chemistry - Trends & Developments, ACS Symposium Series, Eds. R. Teranishi, R.G. Buttery & F. Shahidi, American Chemical Society, Washington (1989), pp. 151-157. 4. Stereochemistry of Carbon Compounds, E. L. Eliel, (McGraw-Hill Book Company, Inc., New York, 1962) 5. Stereochemistry of Organic Compounds, Ernest L. Eliel and Samuel H. Wilen (Wiley, New York, 1994) 6. J.H. van't Hoff, Bull. soc. chim. France, [2]23, 295 (1875) 7. J.A. Le Bel, Bull. soc. chim. France, [2]22, 337 (1874) 8. L. Pasteur, Two lecturesdelivered to the Societe Chimique de Paris, Jan. 20 & Feb. 3, 1860 9. A. Kekule, Ann., 106, 154 (1858) 10. R.S. Cahn, C.K. Ingold & V. Prelog, Tetrahedron, 1, 119 (1961) 11. http://www.chem.ucalgary.ca/courses/350/Carey/Ch07/ch7-6.html 12. Philip Kraft and Riccardo Cadalbert, Constructing Conformationally Constrained Macrobicyclic Musks, Chem. Eur. J., 7, No. 15, 3254 - 3262 (2001) 13. Philip Kraft, Caroline Denis, and Walter Eichenberger, 5,6,7-Trimethylocta-2,5-dien-4-one 2 A Suspected Odorant with Surprising Olfactory Properties, Eur. J. Org. Chem., 2363-2369 (2001) 14. Roman Kaiser & Philip Kraft, Neue und ungewöhnliche Naturstoffe faszinierender Blütendüfte, Chemie in unserer Zeit, 35, No. 1, 8-23 (2001) 15. Markus Gautschi, Jerzy A. Bajgrowicz, and Philip Kraft, Fragrance Chemistry - Milestones and Perspectives, Chimia 55, 379–387 (2001) 16. George Frater, Jerzy A. Bajgrowicz, and Philip Kraft, Fragrance Chemistry, Tetrahedron, 54, 7633-7703 (1998) 17. Philip Kraft, Jerzy A. Bajgrowicz, Caroline Denis and George Frater, Odds and Trends: Recent Developments in the Chemistry of Odorants, Angew. Chem. Int. Ed., 39, 2980-3010 (2000) 18. Philip Kraft and George Frater, Enantioselectivity of the Musk Odor Sensation, Chirality, 13, 388–394 (2001) 19. Georg Frater, Urs Muller, and Philip Kraft, Preparation and Olfactory Characterization of the Enantiomerically Pure Isomers of the Perfumery Synthetic Galaxolide, Helvetica Chimica Acta, Vol. 82, 1656-1665 (1999) 20. J.A. Bajgrowicz and G. Frater, Chiral recognition of sandalwood odorants, Enantiomer, 5(3-4):225-34 (2000) |
Two "chiral" forms
GO TO Ionones, Irones, Damascones & Structurally Related Odorants Acyclics (Alcohols, Esters, Acids, Aldehydes) |
|
|
Ionones, Irones, Damascones & Structurally Related Odorants |
|
Telephone:
01-770-8895111 - Email: leffingwell@mindspring.com
Copyright ©
Leffingwell &
Associates
TERMS OF SERVICE.............PRIVACY POLICY