|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
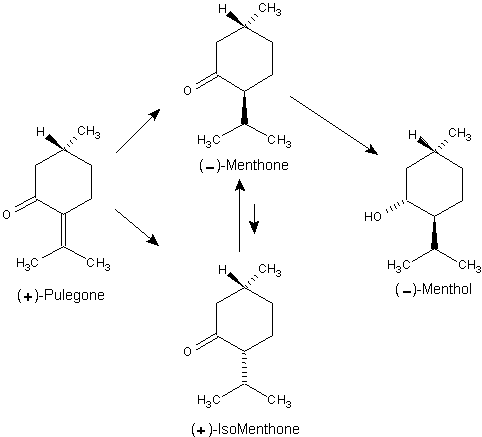
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. The manufacture of (-)-menthol from (+)-pulegone (ex Spanish pennyroyal oil) similarly is dependent on configuration at C-1 in the present material.14 In this process the 4,8-double bond is catalytically hydrogenated and then the mixture of (-)-menthone and (+)isomenthone so produced is reduced by sodium in alcohol to give predominantly (-)-menthol. Reduction of menthones by nascent hydrogen generated in situ is the preferred procedure inasmuch as this system allows epimerization of the isopropyl group and preferential reduction to an all-equatorial substituent system, presumably via the enolate.1 1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993).
|
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY