|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
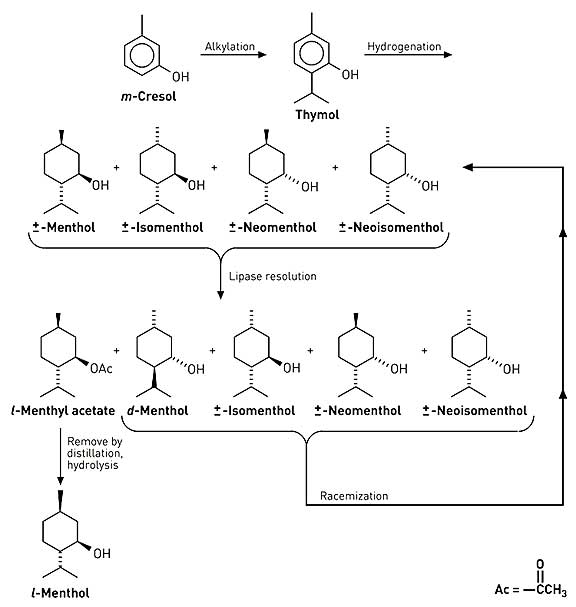
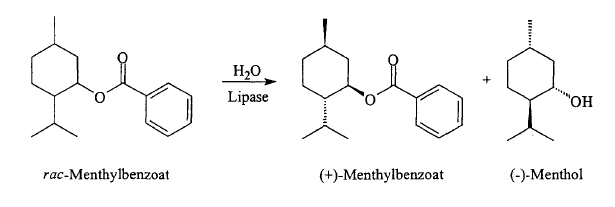
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. Menthol via Lipase Resolution Researchers at CSIR Bio/Chemtek have developed a process to produce l-menthol from the readily available raw material m-cresol. Alkylation of m-cresol generates thymol. Hydrogenation of thymol yields four pairs of racemic diastereomers: menthol, isomenthol, neomenthol, and neoisomenthol. Acylation of this mixture using a stereoselective lipase yields l-menthyl acetate in at least 96% enantiomeric excess (ee). l-Menthyl acetate is separated from the unreacted isomers by distillation. Hydrolysis yields (-)-menthol. The enzymatic resolution has been demonstrated in a continuous process at 1 kg per hour. Enzyme activity is retained even after 2,000 hours of operation. Furthermore, isomerization/racemization of the unreacted isomers regenerates the original mixture of diastereomers, which is routed again to enzyme resolution. Over several cycles, thymol is almost fully converted to l-menthol. This process is the subject of a 2002 patent assigned to AECI which is available for download below. Similarly, Haarmaan & Reimer was recently (July 17, 2002) issued a patent for the resolution of racemic menthol esters (Benzoate, acetate, etc.) using lipases (e.g., Candida rugosa) that provides (-)-menthol with essentially 100% enantioselectivity. This patent is also available for download below. While neither the AECI or H&R process have yet been commercialized, these routes are certainly of high interest. It should be noted that as early as 1968, workers at Takasago had shown that (-)-menthol could be selectively produced by the hydrolysis of racemic esters of menthol using an enzyme carboxylic acid hydrolase.7 1. CHAPLIN, JENNIFER ANN; DICKSON, MELANIE DARYL EVANS; MARAIS, STEPHANUS FRANCOIS; MITRA, ROBIN KUMAR; REDDY, SHAVANI; BRADY, DEAN; PORTWIG, MADRIE; GARDINER, NEIL STOCKENSTROM; MBONISWA, BUTANA ANDREW; PARKINSON, CHRISTOPHER JOHN, PROCESS FOR PREPARING (-)- MENTHOL AND SIMILAR COMPOUNDS, PATENT NO. WO 02/36795 A2 (MAY 10, 2002) 2. BORNSCHEUER, UWE PROF DR; GATFIELD, IAN-LUCAS DR; HILMER, ENS-MICHAEL DR; VORLOVA, SANDRA; SCHMIDT, ROLF PROF DR, Method for preparing D- or L-menthol, Pantent No. EP1223223 (July 17, 2002) 3. Rabiller, C. G.; Koenigsberger, .; Faber, K.; Griengl, H., ENZYMATIC RECOGNITION OF DIASTEREOMERIC ESTERS, Tetrahedron 1990, 46: 12 4231-4240 4. Langrand, Georges; Baratti, Jaquess; Buono, Gerard; Triantaphylides, Christian, LIPASE CATALYZED REACTIONS AND STRATEGY FOR ALCOHOL RESOLUTION, Tetrahedron Lett. 1986, 27: 1 29-32 5. Langrand, Georges; Secchi, Michel; Buono, Gerard; Baratti, Jacques; Triantaphylides, Christian, LIPASE-CATALYZED ESTER FORMATION IN ORGANIC SOLVENTS. AN EASY PREPARATIVE RESOLUTION OF a-SUBSTITUTED CYCLOHEXANOLS, Tetrahedron Lett. 1985, 26: 15 1857-1860 6. Cygler, Miroslaw; Grochulski, Pawel; Kazlauskas, Romas J.; Schrag, Joseph D.; Bouthillier, Francois; et al., A Structural Basis for the Chiral Preferences of Lipases, J.Amer.Chem.Soc. 1994, 116: 8 3180-3186 7. Moroe, T.; Hattori, S; Komatsu, A; Yuzo, Y., Method for the Biochemical Isolation of l-Menthol, U.S. Patent No. 3,607,651 (Sept. 21, 1971) assigne to Takasago Perfumery Co.
|
AECI & Haarmann & Reimer Processes The AECI Process
The H&R Lipase Resolution of Menthyl Esters Process
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY