|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
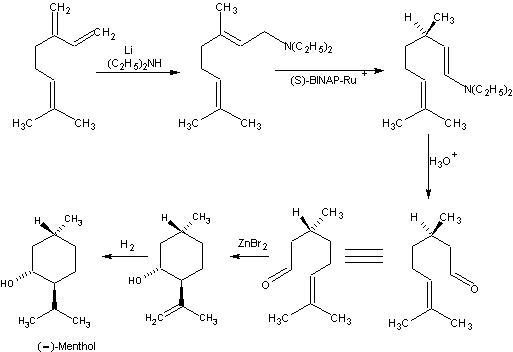
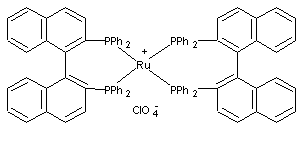
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. In the early 1980's, Takasago developed an elegant synthesis of (-)-menthol from myrcene. Myrcene is converted to diethylgeranylamine by the lithium catalyzed addition of diethylamine. This is then catalytically isomerized to the chiral 3R-citronellal enamine with 96-99% enantiomeric excess. Hydrolysis gives 3R-(+)-citronellal of higher chiral purity than citronellal from citronella oil. The remainder of the synthesis is classical. This is the second major commercial route to (-)-menthol. It should be noted that in the synthetic route shown that zinc bromide is the catylyst used for cyclization of (+)-citronellal to (-) isopulegol. Zinc bromide provides a ratio of (-)-isopulegol to other isopulegol isomers in the ratio of 94 : 6. However, in a very recent patent, Takasago workers have shown that tris(2,6-diarylphenoxy)aluminum catalysts can give a ratio of (-)-isopulegol to other isopulegol isomers in the ratio of 99.7 : 0.3. See reference 8 which you can download. Takasago International Corp. has developed optically active substances by taking advantage of its specialty, the asymmetric synthesis catalysts technology. Specifically, such major products as 1-menthol, and intermediates for agrochemicals and drugs have been commercialized so far. The company is also applying this technology to biodegradable polymers by promoting the development of new asymmetric synthesis catalysts. Although the Iwata plant in Shizuoka is currently the only plant producing optically active substances, there are plans to embark on production in Spain and the United States as well, where the company's synthesis plants are located. The asymmetric synthesis technology of Takasago International selectively produces optically active isomers by using BINAP rhodium catalysts or BINAP ruthenium catalysts. Following the commercialization of 1-menthol in the 1980s, the company is now applying this technology to the production of such intermediates as materials for antibiotics and antibacterial agents. The technology is an ecofriendly synthetic process that enables a synthesis high in optical yield and produces little waste by-products Takasago has been placing "ecofriendly" at the center of its product development policy as well as in the production process and products themselves. For this reason, asymmetric technology is without a doubt the mainstay of the company's operations. Asymmetric technology is also being applied to such compounds as synthetic flavors, intermediates, ceramide, cooling-effect products, warming-effect products and biodegradable polymers. In particular, 3-hydroxy butyric acid monomer is highly expected to have major business uses in the future. Takasago has recently succeeded in developing "SEGPHOS," an asymmetric synthesis ligand, and plans to expand its applications by taking advantage of its asymmetric recognition capacity and catalyst activity, both of which surpass those of BINAP.5,6 Takashi Miura, manager of the fine chemicals research laboratory in Kanagawa, Japan, told Chiral USA 96 in Boston, that the company has produced 1,000 metric tons per year of synthetic menthol by asymmetric hydrogenation since 1984.7 1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993). 3. Shin-ichi Inoue; Hidemasa Takaya; Kazuhide Tani; Sei Otsuka; Tsuneo Sato; Ryoji Noyo, Mechanism of the Asymmetric Isomerization of Allylamines to Enamines Catalyzed by 2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl-Rhodium Complexes, J.Amer.Chem.Soc. 1990, 112 : 12 4897-4905. 4. Kazuhide Tani; Tsuneaki Yamagata; Sei Otsuka; Susumu Akutagawa; Hidenori Kumobayashi; et al., Cationic Rhodium(I) Complex-catalysed Asymmetric Isomerisation of Allylamines to Optically Active Enamines, J.Chem.Soc.Chem.Commun. 1982, 11, 600-601.; Susumu Akutagawa, Enantioselective isomerization of allylamine to enamine: practical asymmetric synthesis of (-)-menthol by Rh--BINAP catalysts, Topics in Catalysis 4 (1997) 3,4, pp. 271-274 5. Japan Chemical Week, October 19, 2000. 6. Dr. Kenzo Sumi, Takasago International Corp., "Derivatives of (4,4’ – Bi – 1,3 – benzodioxole) 5,5’ – diylbis (diphenylphosphine) (SEGPHOS) and Their Application in Ruthenium Catalyzed Asymmetric Hydrogenation", ChiraSource2000, Fifth Annual Conference and Exhibition, October 2-4, 2000, Lisbon, Portugal . 7. Stephen C. Stinson, Technological Innovation Thrives In Fine Chemicals Industry, Chemical & Engineering News, July 15, 1996. 8. 4. HORI YOJI; IWATA TAKESHI; OKEDA YOSHIKI, Process for producing isopulegol , European Patent No. EP1225163 (July 24, 2002) assigned to Takasago International Corporation 9. R. Hopp & B.M. Lawrence, Natural & Synthetic Menthol, in Mint: the genus Mentha, Brian M. Lawrence, Ed., CRC Press, 2006, p. 392-393
|
Takasago Process
The Chiral Rhodium BINAP
Catylyst
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY