|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
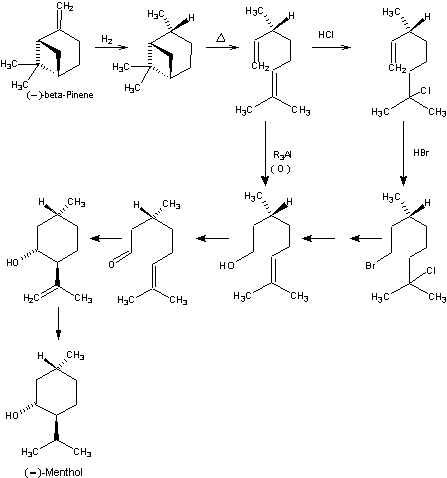
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. An interesting approach in which asymmetric centers are controlled so as to avoid isomeric materials with opposing antipodes in the synthetic sequence was developed by Glidden-Durkee (SCM Corporation) in the early 1960's. (-)-beta-Pinene of very high optical purity occurs as a major constituent in both gum and sulfate turpentine produced in the eastern United States. Typically, these types of turpentine contain 60-65% alpha-pinene and 20-35% (-)-beta-pinene from which the latter is commercially separated by fractional distillation for use as a raw material in resins and for perfume and flavor materials such as geraniol, linalool, citral, nopol, and a multitude of related aromatics. Hydrogenation of (-)-beta-pinene affords predominantly (-)-cis-pinane with the requisite asymmetry at C-1 required for conversion all the way to (-)-menthol. Pyrolysis of (-)-cis-pinane affords optically active 2,6-dimethtyl-2,7-octadiene which can be converted to (+)citronellol by several routes. One procedure involves protection of the 2,3-double bond by reaction with HCl to form 2-chloro-2,6-dimethyl-7-octene followed by anti-Markownikoff addition of HBr. Solvolysis of the intermediate bromo-chloro compound affords a mixture of alpha- and beta-citronellol (either directly or more usually as the ester). [Only the beta-isomer is desired and so the alpha-double bond is subjected to isomerization to the beta position.] Alternatively, direct treatment of 2,6-dimethyl-2,7-octadiene with organoaluminum compounds such as aluminum triisobutyl (or alkyl boranes) and oxidation-hydrolysis affords (+)-beta-citronallal in high yield. This latter sequence offers considerable advantages over the former, provided appropriate safety precautions are employed. Catalytic oxidation of (+)-citronellol gives (+)-citronellal, in good yield, which is then convertible to (-)-menthol by classical procedures. The degree of optical purity of the final product obtained via this procedure is highly dependent on excluding the small percentage of trans-pinane produced in hydrogenation of (-)-beta-pinene. Pyrolysis of this latter material affords antipodal 2,6-dimenthyl-2,7-octadieiie which when carried through the sequence gives (+)-menthol. While seemingly insignificant because of the small percentage generated, this can play a distinct role in the final optical purity of the menthol produced. 1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993).
|
J.P Bain, R.L. Webb & B.J. Kane
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY