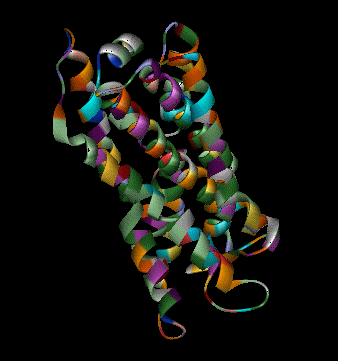|
Leffingwell &
Associates
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com  |
|
General Physiology of Olfaction Trigeminal Sense in the Olfactory Epithelium The Odorant Binding Proteins Odorant receptors The Cellular Membrane G-Protein Coupled Receptors G-Proteins The cAMP Transduction Cascade Ion Protein Channels Other Second Messengers in Olfaction - cGMP, IP3, NO, CO Chemical Olfactory Stimulation - Theories on Olfaction The Steric Theory of Odor The Vibrational Theory of Odor Vibrational Induced Electron Tunneling Spectroscope Theory Ribonucleotides as the Odorant carrier? Recent Events in Olfactory Understanding A Combinatorial Process for odor Interpretation Combinatorial Process Visualization Human Olfactory Receptor Genes Enantiomeric Specificity in the Olfactory bulb |
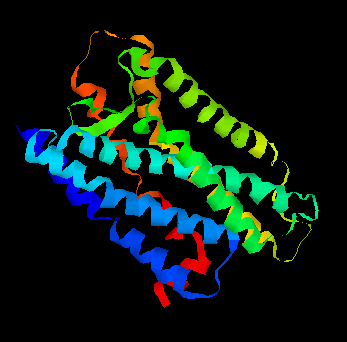
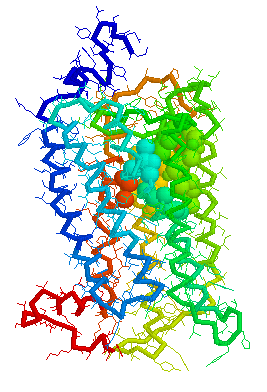
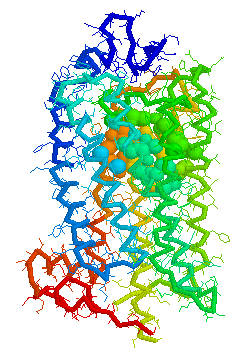
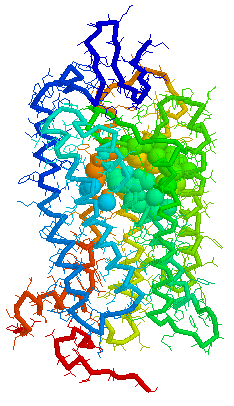
Olfaction-Page 3 John C. Leffingwell, Ph.D. G-Protein Coupled Receptors G-protein coupled receptors are known to have been present in the acoelomate flatworms of the Precambrian era over 800 million years ago. This ancient organism (believed to be the ancestor of all bilaterally symmetric metazoans) contained a surprisingly rich collection of cellular signaling mechanisms.13 G-protein - coupled receptors are a pharmacologically important protein family with approximately 450 genes identified to date. The olfactory receptors are one of the largest groups of G-protein coupled receptors described to date. Olfactory G-protein linked receptors trigger the biochemical synthesis of neurotransmitters, including cAMP and inositol triphosphate, which open cation channels to that ultimately lead to action potentials and signaling.14 In order to visualize the helical nature of the olfactory receptor, we have constructed the following views of the human olfactory receptor OR1.01.05 (Senomyx nomenclature) by homologous modeling against rhodopsin. 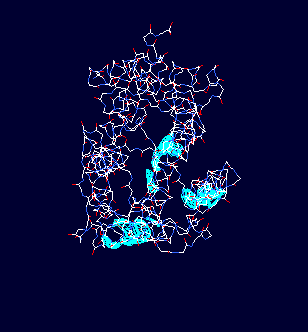
OR1.01.05 Receptor Protein as viewed in
the Swiss-PdbViewer
with
OR1.01.05 Receptor Protein as viewed in Chime
OR1.01.05 Receptor Protein as viewed in Weblab Viewer
The following general principles appear to hold for such receptor proteins:
Recently, Doron Lancet and co-workers have inferred, that for olfactory receptors, the odorant complementarity determining regions reside in the transmembranal segments 3, 4, and 5.14b 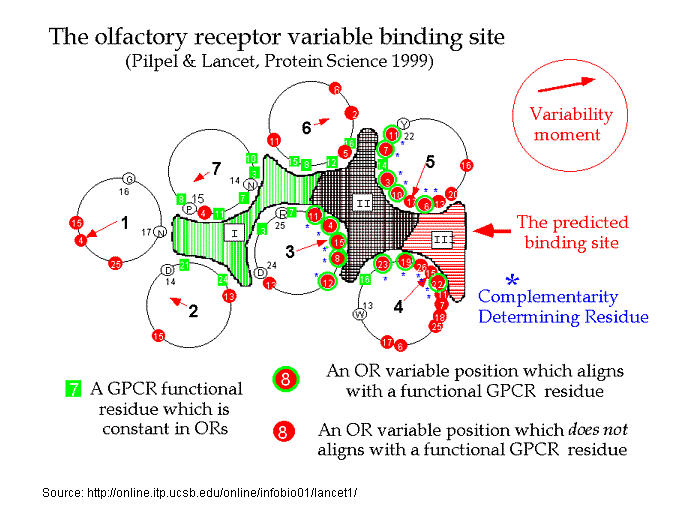 However, Singer14c in an Analysis of the Molecular Basis for Octanal Interactions in the Expressed Rat I7 Olfactory Receptor makes a strong case that octanal binds with OR-I7 in a pocket ~10 Å from the extracellular surface formed by transmembrane domains 3–7. In addition, Goddard, et.al.14d, have modeled the mouse receptor ORL466 (OR S25) and in docking studies predicted the binding pocket for the compounds hexanol and heptanol. Docking results show that TMs 3, 5, and 6 have residues directly involved in binding and that TM4 may have an important role in binding as it packs against TM3 and TM5 and therefore can alter their relative position if key residues of TM4 are mutated. The presence of a critical Lys on TM7 is similar to the related rhodopsin, where Lys-296 (TM7) binds the retinal chromophore14e. Substitutions in this residue may switch receptor specificity toward other functional groups. CastP for determining predicted ligand binding sites Similarly, Leffingwell14f, in a study of the predicted ligand binding sites for certain Human OR's of chromosone 1, has found that the largest cavity (as derived by CastP14i ) in the complimentary region also falls in the transmembrane domains TM 3–7, especially in the region of TM3-TM5-TM6. This is depicted below for Human OR1.04.0612f-g as modeled by Leffingwell. As the CastP technique for determining putative binding cavities and pockets predicts the binding site for retinal in the bovine rhodopsin models 1HXZ (chain A)14g and 1F88 (chain A)14h with an excellent correlation14f to that previously found, CastP14i may provide a simple and fast method for predicting binding sites in olfactory receptors.
Leffingwell has also used the publicly available Human OR PDB models14f to construct homologous models of the Mouse olfactory receptor OR S25 (Mouse ORL466) and the Rat I7 receptor (Rat ORL11) in order to test the CastP methodology for determining binding sites. These receptors have been previously modeled by Singer14c and Goddard, et.al.14d as mentioned above. G-Proteins The following model illustrates both the transmembranal 7-helical receptor protein (at the top) and the G-protein (at the bottom) within the cell. 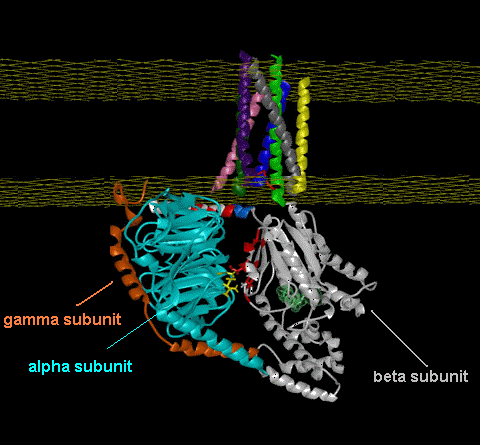 Trimeric G proteins are signaling machines that transduce messages from receptors for extracellular stimuli into cellular responses mediated by effector enzymes or ion channels. Responding to G protein-coupled receptors (GPCRs) for example, odorants, these G proteins relay signals to adenylyl cyclase, phospholipase C, K+ channels, and other effectors. This model (adapted from work at the Bourne lab) of the G protein trimer (bottom), highlights an interaction (red and yellow residues) between the beta and alpha subunits (cyan and gray, respectively) which are postulated to play an important role in mediating GPCR-triggered release of bound GDP (green, near center of alpha subunit). The plasma membrane is indicated by a yellow grid at the top. The gamma subunit is orange. An animated demonstration of the G-protein signaling process is shown in the You Tube video below.
That understanding all of this is "childs play" is also shown in another You Tube video.
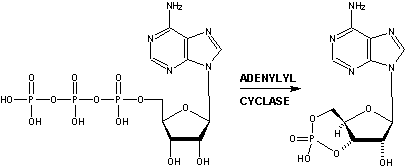
The cAMP Transduction Cascade In simple terms for the cAMP mediated transduction process:
Ion Protein Channels As mentioned, receptor interaction with an odorant triggers activation of the G-protein (i.e., G-olf) that triggers adenylyl cyclase resulting in an increase in intracellular cAMP in the olfactory cells. This sequence of events is referred to as as a multi-step "cascade". The direct activation of a cation permeable channel by cAMP is the final step in producing the odor induced ionic current. In the presence of normal physiological extracellular Ca++, the second messengers (i.e., cAMP) elicits the opening of the channel allowing Ca++ to flow in, and the increase in intracellular Ca++ concentration appears to activate a chloride current that helps depolarize the olfactory cell. Thus the cyclic nucleotide gated channels plus the Cl- evoked whole cell current results in the signal transduction.15-16 Other Second Messengers in Olfaction In some vertebrate species, odorants have been shown to elicit rapid increases in IP3, as well as in cAMP, implicating that two separate signal transduction pathways exist in olfactory neurons (Breer and Boekhoff, 1992; Schild and Restrepo, 1998). The odorants citralva and eugenol appear to increase cAMP, whereas some other odorants such as lyral, lilial, and ethyl vanillin have been shown to elicit IP3 increases in biochemical assays (Breer and Boekhoff, 1992; Schild and Restrepo, 1998). IP3 (Inositol TriPhosphate) Another second messenger, Inositol TriPhosphate (IP3) is also known to actively participate in olfactory transduction within certain mammals. While the general cascade process is similar to that described for cAMP above, chemically it is quite different. There is evidence that another category of G-protein is involved in the activation of the intracellular membrane-bound enzyme phospholipase C (PLC). PLC hydrolyses a lipid phosphatidylinositol-4,5-bisphosphate (PIP2) in the plasma membrane, producing inositol trisphosphate (IP3) and diacyl glycerol (DAG). In 1998, Breer and co-workers18 identified particular individual G protein subtypes that were activated upon stimulation of isolated olfactory cilia by chemically distinct odorants. These results indicate that different subsets of odorants selectively trigger distinct reaction cascades and provide evidence for dual transduction pathways in olfactory signaling (i.e. a cAMP path and a IP3/DAG path).19 Both IP3 and DAG can act directly on ion channels and also on intracellular Ca2+ levels. It thus appears that both cAMP and IP3/DAG systems may coexist in the same cell and may be activated by different odorants.20 They may even have anti effects, since a rise in calcium levels might activate Ca2+-dependent K+ channels which may hyperpolarize the cell and slow or terminate signaling. cGMP Recent evidence that the cAMP transduction cascade is mediated by another cyclic nucleotide cGMP (cyclic Guanosine MonoPhosphate) has been published.17 It appears that odorants cause a delayed and sustained elevation of cGMP. It has been suggested that at least part of this response is due to activation of one of two ciliar receptor guanylyl cyclases by calcium and a guanylyl cyclase activating protein (G-cap). The cGMP formed serves to augment the cAMP signal by activation of adenylyl cyclase. cAMP, which in turn, negatively regulates guanylyl cyclase, limiting the cGMP signal. [See also the role of Carbon monoxide]. The Roles of Nitric Oxide and Carbon Monoxide Both Nitric oxide and Carbon monoxide also appear to play roles in the olfactory system. Nitric oxide (NO) has been implicated in synaptic plasticity in other regions of the brain as a result of its modulation of cyclic GMP levels. In sheep, the neuronal enzyme nitric oxide synthase (nNOS) is expressed in both mitral and granule cells, whereas the guanylyl cyclase subunits that are required for NO stimulation of cGMP formation are expressed only in mitral cells. Nitric oxide therefore seems to act as a retrograde and/or intracellular messenger, being released from both mitral and granule cells to potentiate glutamate release from mitral cells by modulating cGMP concentrations. Kendrick21 therefore has proposed that the resulting changes in the functional circuitry of the olfactory bulb underlie the formation of olfactory memories . This is consistent with the report of Okere, et.al.22, who demonstrated that exogenous administration of nitric oxide into the female mouse accessory olfactory bulb (whose synaptic activities are modified by pheromonal inputs after mating) can induce a pheromone-specific olfactory memory without mating. In addition, data suggests a prominent function for NO in activity-dependent establishment of connections within both developing and regenerating olfactory neurons.23 Similarly, results indicate that Carbon monoxide (CO) may serve as a gaseous neuronal messenger linked to cyclic GMP production suggesting its involvement in the developmental processes of the olfactory receptor neuron.24 Further, it has been found that endogenous CO/cGMP signals contribute to olfactory adaptation and underlie the control of gain and sensitivity of odor transduction. The findings offer a mechanism by which a single, brief odor stimulus can be translated into long-lasting intracellular changes that could play an important role in the perceptual adaptation to odors, and explain the long-standing puzzle that the olfactory CNG channels can be gated by both cAMP and cGMP.25 _____________ 13. Rice, Ken, The Evolution and Classification of G-protein-coupled Receptors (1998) 14. Anholt, R.R., Molecular neurobiology of olfaction, Crit. Rev. Neurobiol. 7(1): 1-22 (1993) 14a. Baldwin, J.M., The probable arrangement of the helices in G protein-coupled receptors., EMBO J. 12, 1693-1703 (1993). 14b. Pilpel, Y. and D. Lancet , The variable and conserved interfaces of modeled olfactory receptor proteins., Protein Sci., May;8(5):969-77 (1999). 14c. Singer, Michael S., Analysis of the Molecular Basis for Octanal Interactions in the Expressed Rat I7 Olfactory Receptor, Chem. Senses 25: 155-165, (2000) 14d. Floriano WB, Vaidehi N, Goddard WA 3rd, Singer MS, Shepherd GM., Molecular mechanisms underlying differential odor responses of a mouse olfactory receptor. Proc Natl Acad Sci U S A, Sep 26;97(20):10712-6 (2000). 14e. Singer, M. S., Weisinger-Lewin, Y., Lancet, D. & Shepherd, G. M., Positive selection moments identify potential functional residues in human olfactory receptors., Recept. Channels 4, 141-147 (1996) 14f. Leffingwell & Associates, Canton, GA, Press Release, February 13, 2002; Leffingwell, J.C. 14g. Teller, D. C., Okada, T., Behnke, C. A., Palczewski, K., Stenkamp, R. E.: Advances in Determination of a High-Resolution Three-Dimensional Structure of Rhodopsin, a Model of G-Protein-Coupled Receptors (Gpcrs) Biochemistry 40 pp. 7761 (2001) 14h. Palczewski, K., Kumasaka, T., Hori, T., Behnke, C. A., Motoshima, H., Fox, B. A., Le Trong, I., Teller, D. C., Okada, T., Stenkamp, R. E., Yamamoto, M., Miyano, M.: Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor Science 289 pp. 739 (2000) 14i. Edelsbrunner H, Facello M, Liang J., On the definition and the construction of pockets in macromolecules. Disc. Appl. Math. 88:83-102 (1998).; Liang J, Edelsbrunner H, Woodward C., Anatomy of protein pockets and caviteis: measurement of binding site geometry and implications for ligand design. Protein Science. 7:1884-1897 (1998).; J. Liang, H. Edelsbrunner, P. Fu, P.V. Sudhakar and S. Subramaniam., Analytical shape computing of macromolecules I: molecular area and volume through alpha shape. Proteins. 33, 1-17 (1998).; Liang J, Edelsbrunner H, Fu P, Sudhakar PV, Subramaniam S., Analytical shape computation of macromolecules. II. Identification and computation of inaccessible cavities in proteins. Proteins: Struct. Funct. Genet. 33:18-29 (1998 ). 15. Kurahashi, T. and K.W. Yau, Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells, Nature, May6, 363(6424):71-74 (1993) 16. Schild, D. and D. Restrepo, Transduction mechanisms in vertebrate olfactory receptor cells, Physiol. Rev., 78(2):429-466 (1998) 17. Moon, C., P. Jaberi, A. Otto-Bruc, W.Behr, K. Palczewzki and G.V. Ronnett, Calcium-sensitive particulate guanylyl cyclase as a modulator of cAMP in olfactory receptor neurons, J. Neurosci., 18(9): 3195-3205 (1998). 18. Jia, C. P. and M. Halpern, Subclasses of vomeronasal receptor neurons: Differential expression of G proteins (Gia2 and Goa) and segregated projections to the accessory olfactory bulb. Brain Res. 719, 117-128 (1996); and Schandar M., K.L. Laugwitz, I. Boekhoff, C. Kroner, T. Gudermann, G. Schultz, H. Breer, Odorants selectively activate distinct G protein subtypes in olfactory cilia., J. Biol. Chem., Jul 3; 273(27):16669-16677 (1998); Breer H, Boekhoff I, Second messenger signalling in olfaction.Curr Opin Neurobiol 2:439–443 (1992) ; Schild D, Restrepo D, Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev 78:429–466 (1998). 19. See also http://www.monell.org/neuroscience.htm 20. Kashiwayanagi, M., K. Shimano, K. Kurihara, Responses of single bullfrog olfactory neurons to many odorants including cAMP-dependent and independent odorants: existence of multiple receptors in single neuron. Brain Res. 738, 222-228 (1996). 21. Kendrick K.M., R. Guevara-Guzman, J.Zorrilla, M.R. Hinton, K.D. Broad, M. Mimmack, S. Ohkura, Formation of olfactory memories mediated by nitric oxide., Nature, Aug 14;388(6643):670-674 (1997) 22. Okere, C.O., H. Kaba, T. Higuchi, Formation of an olfactory recognition memory in mice: reassessment of the role of nitric oxide., Neuroscience, Mar;71(2):349-354 (1996) 23. Roskams A.J., D.S. Bredt, T.M. Dawson, G.V. Ronnett, Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons., Neuron, Aug;13(2):289-299 (1994). 24. Ingi T, Ronnett GV, J Direct demonstration of a physiological role for carbon monoxide in olfactory receptor neurons. Neurosci., Dec;15(12):8214-8222 (1995) 25. Zufall, F., T. Leinders-Zufall, Identification of a long-lasting form of odor adaptation that depends on the carbon Monoxide/cGMP second-messenger system., J. Neurosci., Apr 15;17(8):2703-2712 (1997) ...Back to Page 1 on Olfaction.........Back to Page 2 on Olfaction....... Forward to Page 4 on Olfaction......Home
|
|
|
Telephone: 01-770-8895111 - Email: leffingwell@mindspring.com
TERMS OF SERVICE.............PRIVACY POLICY