Leffingwell &
Associates
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Leffingwell &
Associates
|
 |
by John C. Leffingwell & Diane Leffingwell
(A version of this appeared in Perfumer & Flavorist, Vol. 39, No. 1, 26-37: 2014)
|
|
|
|
|
|
57817-89-7 |
Glucosyl steviol glycosides; Stevia extract, enzymatically modified |
Natural low calorie sweetener and flavor enhancer. Glucosyl steviol glycosides is a complex mixture of glucosides prepared by enzymatically modifying stevia extract to increase/change the degree of glycosylation. In United States Patent 8318459 (Nov. 27, 2012) by Avetik Markosyan entitled Glucosyl stevia composition (assigned to PureCircle USA) it is demonstrated that improved sweetness profiles can be achieved. In WIPO Patent Application WO2012128775 by Siddhartha Purkayastha entitled GLUCOSYLATED STEVIOL GLYCOSIDE COMPOSITION AS A TASTE AND FLAVOR ENHANCER (assigned to PureCircle USA) and WIPO Patent Application WO2012129451 by Siddhartha Purkayastha entitled GLUCOSYLATED STEVIOL GLYCOSIDE COMPOSITION AS A FLAVOR MODIFIER (assigned to PureCircle USA) the used in modifying the flavor profiles of various products is described. |
EXTRACT GLUCOSIDES |
|
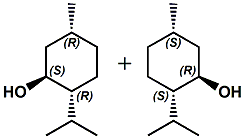
3623-52-7 |
Isomenthol; dl-Isomenthol; (±)-Isomenthol |
dl-Isomenthol alone is considerably less cooling than (-)-Menthol and posesses a somewhat musty, woody camphoric odor. But, in United States Patent 8496950 (July 30, 2013) by Klaus Sorge, Hubert Loges, Arnold Machinek, Ulrike Simchen & Horst Surburg entitled MIXTURE CONTAINING MENTHOL assigned to Symrise the use of dl-Isomenthol in various menthol mixtures has been shown to lower the melting point of such mixtures. In addition, at the levels used the organoleptic perception is acceptable or improved. |
 |
|
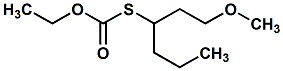
1241905-19-0 |
Ethyl S-(1-methoxyhexan-3-yl)carbonothioate; O-ethyl S-(1-methoxyhexan-3-yl) carbonothioate |
Sulfury, blackcurrant, tropical, roasted coffee; effective in coffee This material is the subject of WIPO
Patent Application WO/2010/115920 entitled CARBONOTHIOATES
AS FLAVOURS AND FRAGRANCES (10/14/2010) by Klaus
Gassenmeier, assigned to
Givaudan. |
 |
|
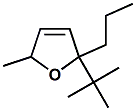
871465-49-5 |
Cassyrane®; 2-tert-Butyl-5-methyl-2-propyl-2,5-dihydrofuran |
In United States Patent 7,632,790 (Dec. 15, 2009) by Philip Kraft assigned to Givaudan, it is described as: Odor description: Blackcurrant, natural, rich, eucalyptus buds, anis, buchu leaves, slightly green. Descriptions of the enantiomers can be found at http://www.leffingwell.com/chirality/cassyranes.htm |
 |
|
83861-74-9 |
1,5-Octadien-3-ol; Octa-1,5-dien-3-ol |
Mixture of E/Z stereoisomers: with 60-90
% (E) Boelens indicates: dusty, somewhat
earthy, mushroom-like odor (PMP 2001)
|
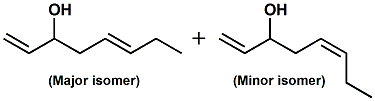 |
|
1006684-20-3 |
2-Mercapto-4-heptanol |
Flavor description: fruity, tropical,
guava, watercress, vegetal (at 0.5 ppm in 5% sugar solution)
The flavor description is for the diastereomeric mixture. |
 |
|
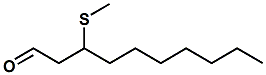
1256932-15-6 |
3-(Methylthio)decanal |
At the time of this writing we have no definitive information on the flavor of this material. From the structure, we would anticipate that the organoleptic character would approximate "roasted, fatty, aldehydic". Primarily finds use in savory, meat & fish flavors. |
 |
|
13552-95-9 |
(4Z,7Z)ü-Tridecadienal |
(4Z,7Z)ü-Tridecadienal is a fatty flavor aldehyde originally discovered by Lever Bros. as useful in fatty, savory flavors. In Great Britain Patent 1,034,352, Dutch Pat. No. 139,102 and Swiss Pat No. 471545 it is reported in the claims that both cis-4-decenal and cis,cis-4,7-tridecadienal and other polyunsaturated aldehydes containing 11-17 carbon atoms are suitable for imparting a savory aroma that enhanced chicken meat flavor to foodstuffs. In Japanese Patent JP2012034662 (Feb. 23, 2012) by Tsukasa Saito et al. entitled DRIED FISH FLAVOR IMPROVING AGENT (assigned to T. Hasegawa) the use of 2,4,7-tridecatrienal and/or 4,7-tridecadienal for producing dried fish flavors (specifically, dried bonito) is disclosed. At low levels they can also impart umami effects. |
 |
|
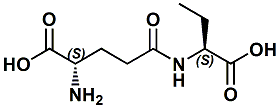
16869-42-4 |
L-gamma-Glutamyl-L-2-aminobutyric acid; gamma-Glu-Abu |
A Kokumi imparting agent. In United States Patent Application 20120034364 (Feb. 9, 2012) by Fumie Futaki, Reiko Yasuda, Seiichi Sato, Takashi Miyaki, Naohiro Miyamura & Yuzuru Eto entitled USE OF PEPTIDES FOR IMPARTING KOKUMI, is described a substance that provides a kokumi-imparting agent consisting of gamma-Glu-Abu (L-gamma-glutamyl-L-2-amino-butyric acid). The kokumi-imparting agent of the present invention or gamma-Glu-Abu can also be used in combination with at least one additional ingredient for seasonings selected from the group consisting of amino acids such as sodium glutamate (MSG), nucleic acids such as inosine monophosphate (IMP), inorganic salts such as sodium chloride, organic acids such as citric acid, and various kinds of yeast extracts to thus provide a favorable seasoning composition which is improved in its kokumi, as compared with those obtained by the use of such additional ingredients for seasonings individually. |
 |
|
38837-71-7 |
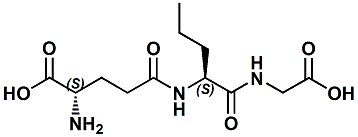
Glutamyl-norvalyl-glycine; N-(N-L-gamma-Glutamyl-L-norvalyl)-glycine; L-Glutamyl-L-norvalyl-glycine; gamma-Glu-Nva-Gly |
A Kokumi flavor enhancer In WIPO Patent Application WO2011081186 (July 7, 2011) by Takashi Miyaki et al. entitled FLAVOR-ENRICHING AGENT it is disclosed that gamma-Glu-Nva-Gly (L-gamma-glutamyl-L-norvaline-glycine) exhibits a potent flavor-enriching action, particularly with respect to middle tastes, and exhibits an improved stability. |
 |
|
71133-09-0 |
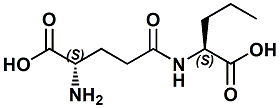
Glutamyl-norvaline; L-gamma-Glutamyl-L-norvaline N-(N-L-gamma-Glutamyl)-L-norvaline; L-Glutamyl-L-norvaline; gamma-Glu-Nva |
A Kokumi flavor enhancer In United States Patent 8541379 (Sept.24, 2013) by Takashi Miyaki et al. entitled Kokumi-imparting agent (assigned to Ajinomoto) wherin gamma-Glu-Nva (L-gamma-glutamyl-L-norvaline) is the subject compound. Examples are given of the kokumi effect of L-Glutamyl-L-norvalyline wherein the intensity of the kokumi-imparting activity observed for each test compound was determined as a value observed when blending 0.00001 to 0.5 g/dL of the corresponding test compound with the distilled water containing sodium glutamate (0.05 g/dL), inosinic acid monophosphate (0.05 g/dL), and sodium chloride (0.5 g/dL). The Kokumi effect was effective at a dose level of 0.0001 g/dl which was a significantly lower dose level than a number of other kokumi compounds tested. |
 |
|
851670-40-1 |
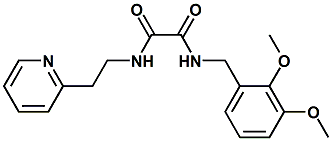
N-(2,3-Dimethoxybenzyl)-N'-(2-(pyridin-2-yl)ethyl)oxalamide; N1-(2,3-dimethoxybenzyl)-N2-(2-(pyridin-2-yl)ethyl)oxalamide |
Savory; umami flavor enhancer This material is disclosed in United States Patent 7476399 (01/13/2009) entitled "Flavors, flavor modifiers, tastants, taste enhancers, umami or sweet tastants, and/or enhancers and use thereof " by Catherine Tachdjian et al. (assigned to Senomyx) - and was identified as a umami compound. |
 |
|
917750-72-2 |
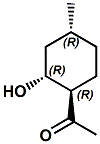
1-(2-Hydroxy-4-methylcyclohexyl)ethanone |
In United States Patent 8,071,531 (Dec. 6, 2011) by Takashi Aida & Kenya Ishida entitled Flavor and fragrance composition (assigned to Takasago) this material is decribed as having an acetophenone-like odor with sweetness and having a "cool and refreshing feeling equivalent to 10 ppm of menthol at a concentration of 50 ppm; appearance of refreshing feeling is relatively rapid with clear refreshing feeling without unpleasant taste" in aqueous solution.. Synergetic Effect with Menthol:
|
 |
|
62439-41-2 |
(±)-6ü-Methoxyü-2,6ü-dimethylheptanal; Methoxymelonal |
Citrus, floral, fruity notes with a watery melon character First reported in United States Patent 4311617 (Jan. 19, 1982) by Hifzur R. Ansari, Benjamin O. Isaac & Horst R. Wagner, entitled Perfumery compositions. It has long been used in fragrances. Michael Zviely (Perfumer & Flavorist, Vol. 35, October 2010, pp. 22-23) reports it has "a light floral odor, slightly fruity note with a watery citrus character. It is fresh, herbaceous and slightly fruity with melon connotations". |
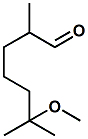 |
|
68973-20-6 |
3,5-Undecadien-2-one |
Odor as described by Sungim Im, Fumiyo
Hayakawa and Tadao Kurata, Identification and Sensory
Evaluation of Volatile Compounds in Oxidized Porcine Liver,
J. Agric. Food Chem., 2004, 52 (2), pp
300–305: 3,5-undecadien-2-one was also described as "Fatty, fried" by H. T. Badings, Neth. Milk Dairy J., 24, 147-257 (1970) as referenced by David A. Forss, ODOR AND FLAVOR COMPOUNDS FROM LIPIDS in Progress in the Chemistry of Fats and other Lipids, Pergamon Press, 1975, p. 236) |
 |
|
91212-78-1 |
(±)-2,5-Undecadien-1-ol; Undeca-2,5-dien-1-ol |
The author is unfamiliar with this
material, but by analogy expects it to have a somewhat
fatty, waxy
character. (Z,Z)-2,5-undecadien-1-ol is found in Noni (Morinda citrifolia L.) |
|
|
54717-17-8 |
Triethylthialdine; 2,4,6-Triethyl-1,3,5-dithiazinane; 5,6-Dihydro-2,4,6-triethyl-4H-1,3,5-dithiazine |
Sulfurous, fried onion, leek, meaty, savory, seafood Boelens indicates - narcotic, sulfurous- and amine-like; seafood note (FRM 2001) Treatt indicates - Sulphurous, savoury; ‘cooked leek’. Used in flavours for oils and fats at 0.35-3.5 ppm; in meat and dairy flavours for snack foods and sauces at 3-6 ppm; and in soups and seasonings, at 4-8 ppm. Also described as a powerful "fried onion" note. Also described as weak raw pumpkin, white part of Allium plants (at pH 7.3) and green, leek at lower pH by Tetsuo Kawai, J. Agric. Food Chem. 1985, 33, 393-397. |
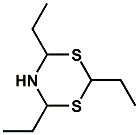 |
|
35852-42-7 |
4-methylpentyl 4-methylpentanoate; 4-methylpentyl 4-methylvalerate; Isohexyl isohexanoate |
Sweet, fruity, waxy, soapy, slightly herbal Considered sensorially relevant to capsicum peppers. Primarily used in fruit flavors |
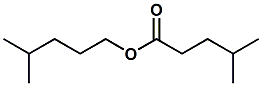 |
|
65405-77-8 |
cis-3-Hexenyl salicylate; (Z)-3-Hexenyl salicylate; (Z)-Hex-3-enyl salicylate |
Sweet, green, floral, woody, balsamic Used as a modifier in floral & fruit flavors |
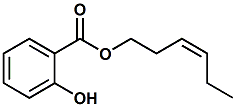 |
|
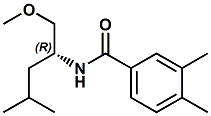
851669-60-8 |
(R)-N-(1-methoxy-4-methylpentan-2-yl)-3,4-dimethylbenzamide |
Savory; umami flavor enhancer This material is disclosed in United States Patent 7476399 (01/13/2009) entitled "Flavors, flavor modifiers, tastants, taste enhancers, umami or sweet tastants, and/or enhancers and use thereof " by Catherine Tachdjian et al. (assigned to Senomyx) - and was identified as a umami compound. |
 |
|
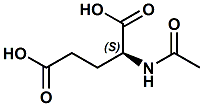
1188-37-0 |
Acetyl glutamate; N-Acetyl-L-glutamate; N-Acetyl-L-glutamic acid; alpha-(N-Acetyl)-L-glutamic acid |
Flavor enhancer, umami like In WIPO Patent Application WO2013010991 (Jan. 24, 2013) by Xiaogen Yang et al. entitled FLAVOUR MODIFYING COMPOUNDS (assigned to Givaudan) it is disclosed that N-acetyl glutamic acid can modify the taste or flavour, in particular the salt taste, umami taste, or savoury flavour, of a flavour composition or consumable product . |
 |
|
504-63-2 |
1,3-Propanediol; 1,3-Propylene glycol; Zemea® propanediol |
A certified natural product replacement for 1,2-propylene glycol; can be used to replace glycols such as propylene glycol (1,2-propanediol), butylene glycol (1,3-/1,4-butanediol) or glycerin. |
|
|
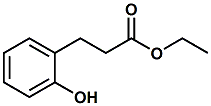
20921-04-4 |
Ethyl melilotate; Ethyl 3-(2-hydroxyphenyl)propanoate; Ethyl 3-(2-hydroxyphenyl)propionate |
Adds sweetness, extends vanilla character, sweet-cream-vanilla notes. In WIPO Patent Application WO2013000595 (Jan. 3, 2013) by Estelle Delort & Erik Decorzant entitled FLAVORING COMPOUND (assigned to Firmenich) Ethyl 3-(2-hydroxyphenyl)propanoate is disclosed as a flavoring ingredient to impart or reinforce a coumarine and/or vanilla tonality. |
 |
|
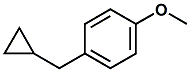
16510-27-3 |
Toscanol®; 1-(Cyclopropylmethyl)-4-methoxybenzene; 1-Cyclopropylmethyl-4-methoxybenzene; p-(Cyclopropylmethyl)anisole |
Strong anisic, estragole, anethole, sassafras, cresolic Givaudan indicates for Toscanol® -
Odor: Anisic, Green, Liqueur like,
Herbaceous See also - United States Patent 7704942 (April 27, 2010) entitled Fragrance and flavour compositions by Andreas Goeke (assigned to Givaudan). |
 |
|
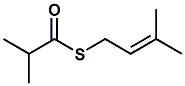
53626-94-1 |
S-Prenyl thioisobutyrate; S-(3-Methylbut-2-enyl) 2-methylpropanethioate |
Boelens indicates - sulfurous, fruity,
tropical fruit connotation, durian, passion fruit odor (PMP
2001) Found in Agathosma oils related to buchu. |
 |
|
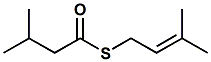
75631-91-3 |
S-Prenyl thioisovalerate; S-3-Methylbut-2-enyl 3-methylbutanethioate; S-Prenyl thioisopentanoate; S-(3-methylbut-2-en-1-yl) 3-methylbutanethioate |
Sulfurous, fruity, tropical fruit
connotation, durian, passion fruit
notes. Found in Agathosma oils related to buchu. |
 |
|
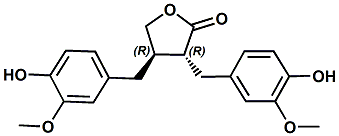
580-72-3 |
(-)-Matairesinol; (8R,8'R)-(-)-Matairesinol; (3R,4R)-Dihydro-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]-2(3H)-furanone; (3R,4R)-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one |
Reduces bitterness and astringency. In European Patent Application EP2517574 (Oct. 31, 2012) by Michael Backes et al. entitled Specific vanillyl lignanes and their use as taste enhancers (assigned to Symrise) the use of (-)-Matairesinol has been shown to reduce both bitter impressions and astrigency at levels of about 25-100 ppm. |
 |
|
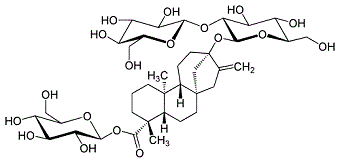
57817-89-7 |
Stevioside; Steviosin; (4,alpha)-13-[(2-O-beta-D-Glucopyranosyl-alpha-D-glucopyranosyl)oxy]kaur-16-en-18-oic acid beta-D-glucopyranosyl ester |
Natural low calorie sweetener Steviol glycosides are responsible for the sweet taste of the leaves of the stevia plant (Stevia rebaudiana Bertoni). These compounds range in sweetness from 40 to 300 times sweeter than sucrose. The four major steviol glycosides found
in the stevia plant tissue
are: |
 |
|
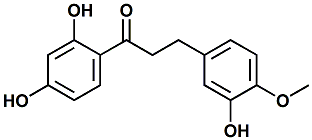
50297-39-7 |
1-(2,4-Dihydroxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)propan-1-one |
Sweetness enhancer, bitterness reducer. In United States Patent Application 20080113073 (May 15, 2008) by Jakob Ley, Gunter Kindel, Susanne Paetz & Gerhard G.E. Krammer entitled HYDROXYDEOXYBENZOINS AND THE USE THEREOF TO MASK A BITTER TASTE, this material was demonstrated to enhance sweetness of sucrose and reduce bitterness (of caffeine). |
 |
|
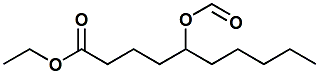
1367348-37-5 |
Ethyl 5-(formyloxy)decanoate; Ethyl 5-formyloxydecanoate |
delta-decalactone precursor; dairy like This material forms delta-decalactone
when heated or under enzymatic
conditions. |
 |
|
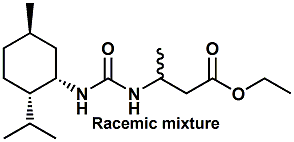
1160112-20-8 |
3-[3-(2-Isopropyl-5-methyl-cyclohexyl)ureido]butyric acid ethyl ester; Ethyl 3-(3-(menthyl)ureido)butyrate; Ethyl 3-(3-(2-isopropyl-5-methylcyclohexyl)ureido)butanoate |
Salty, metallic, umami In United States Patent Application
20090311401 (Dec. 17, 2009) by Jakob Peter Ley, Heiko
Oertling, Michael Backes & Tobias Vossing entitled
Neo-Menthyl Derivatives as Flavor Materials (assigned to
Symrise) discloses a series of ureas, thioureas, carbamates,
thiocarbamates and guanidines based on the neomenthyl
structure as flavor materials or flavor material mixtures
for producing, imparting, modifying and/or enhancing savory
flavor notes. |
 |
|
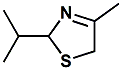
67936-13-4 |
2-Isopropyl-4-methyl-3-thiazoline; 2-Isopropyl-4-methyl-2,5-dihydrothiazole; 2,5-dihydro-2-isopropyl-4-methylthiazole |
Flavor enhancer for fruit flavors, brown flavors & Savory flavors In European Patent Application EP2289351
(Mach 2, 2011) by Kathryn A. Bardsley, Kenneth J. Kraut,
Linda Psota-Kelty & LaurenceTrinnaman entitlted Use of
thiazoline compounds in flavor applications (assigned to
IFF) the flavor use of 2-Isopropyl-4-methyl-3-thiazoline is
disclosed. |
 |
|
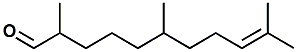
141-13-9 |
Adoxal (Givaudan); 2,6,10-trimethyl-9-undecenal |
Fresh, aldehydic, powerful, floral, rosy, ozonic-marine. Givaudan indicates: Olfactive note -
Fresh, Aldehydic, Powerful, Floral, Rosy,
Marine |
 |
|
851768-51-9 |
5-Mercapto-5-methyl-3-hexanone; Sauvignone 100 (Takasago) |
Sparkling citrus, grape, fruity, watery, tropical notes Takasago indicates - Odor Description:
Sparkling Citrus,
Grape |
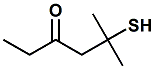 |
|
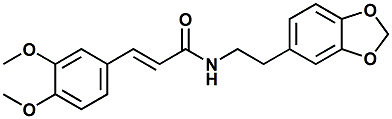
125187-30-6 |
Rubescenamine; (E)-N-[2-(1,3-Benzodioxol-5-yl)ethyl]-3-(3,4-dimethoxyphenyl)prop-2-enamide |
Umami flavor enhancer In United States Patent Application
20120308703 (Dec. 6, 2012) by Jakob Peter Ley,Katharina
Reichelt, Susanne Paetz, Michael Backes & Katja Obst
entitled CINNAMAMIDES AS SAVORY FLAVORINGS (assigned to
Symrise), Rubescenamine (along with a number of structurally
similar compounds) is disclosed as an excellent savory
flavor ingredient.
|
 |
|
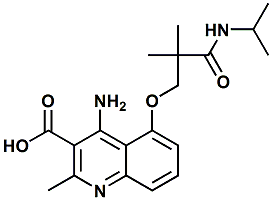
1359963-68-0 |
4-amino-5-(3-(isopropylamino)-2,2-dimethyl-3-oxopropoxy)-2-methylquinoline-3-carboxylic acid; 5-(2-(isopropylcarbamoyl)-2-methylpropoxy)-4-amino-2-methylquinoline-3-carboxylic acid |
Sweetness enhancer / modifier This material is covered by the claims in Senomyx United States Patent Application 20110245353 (Oct. 6, 2011) entitled Sweet Flavor Modifier and specifically mentioned in Senomyx United States Patent Application 20120041078 (Feb. 16, 2012) entitled METHOD OF IMPROVING STABILITY OF SWEET ENHANCER AND COMPOSITION CONTAINING STABILIZED SWEET ENHANCER |
 |
|
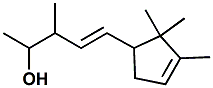
67801-20-1 |
Ebanol®; 3-Methyl-5-(2,2,3-trimethylcyclopent-3-en-1-yl)pent-4-en-2-ol |
Sandalwood, Musk aspect, Powerful Givaudan indicates - Olfactive note:
Sandalwood, Musk aspect,
Powerful The enantiomers of Ebanol are described at http://www.leffingwell.com/chirality/ebanol.htm |
 |
|
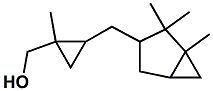
198404-98-7 |
Javanol®; (1-Methyl-2-(1,2,2-trimethylbicyclo[3.1.0]-hex-3-ylmethyl)cyclopropyl)methanol (Mixture of diastereoisomers) |
Sandalwood, creamy, rosy, powerful odor This material is one of the most powerful and best of the sandalwood replacements. Givaudan indicates - Olfactive note:
Sandalwood, Creamy, Rosy,
Powerful |
 |
We look forward to receiving additional insights and descriptors for these materials - email: leffingwell@mindspring.com