|
|
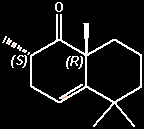
(+)-(2S,8aR)-2,5,5,8a-tetramethyl-3,5,6,7,8,8a-hexahydronaphthalen-1(2H)-one
- The two enantiomers of this compounds, namely
(2S,8aR)-2,5,5,8a-tetramethyl-3,5,6,7,8,8A-hexahydro-1(2H)-naphthalenone
and
(2R,8aS)-2,5,5,8a-tetramethyl-3,5,6,7,8,8A-hexahydro-1(2H)-naphthalenone,
although having organoleptic properties similar
to the racemate, display different intensities,
the (2S,8aR) enantiomer being more powerful than
the (2R,8aS) enantiomer.
The racemate
is described as having an odour with woody
(earthy-patchouli type), powdery and ionone
(violet) notes. In fact, this unique combination
of odour notes can give the impression of a nice
woody, irone and slightly patchouli
scent.
=
(2S,8aR)-2,3,6,7,8,8a-hexahydro-2,5,5,8a-tetramethylnaphthalen-1(5H)-one
Ref: Charles
Fehr & Iris Farris; Naphthalenone Derivative
with Powdery-Ionone Type Odors, United States
Patent Application 20080214419 (September 4,
2008)
|
|
|
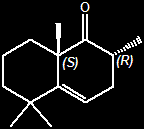
(-)-(2R,8aS)-2,5,5,8a-tetramethyl-3,5,6,7,8,8A-hexahydro-1(2H)-naphthalenone
- The two enantiomers of this compounds, namely
(2S,8aR)-2,5,5,8a-tetramethyl-3,5,6,7,8,8A-hexahydro-1(2H)-naphthalenone
and
(2R,8aS)-2,5,5,8a-tetramethyl-3,5,6,7,8,8A-hexahydro-1(2H)-naphthalenone,
although having organoleptic properties similar
to the racemate, display different intensities,
the (2S,8aR) enantiomer being more powerful than
the (2R,8aS) enantiomer.
The racemate
is described as having an odour with woody
(earthy-patchouli type), powdery and ionone
(violet) notes. In fact, this unique combination
of odour notes can give the impression of a nice
woody, irone and slightly patchouli
scent.
=
(2R,8aS)-2,3,6,7,8,8a-hexahydro-2,5,5,8a-tetramethylnaphthalen-1(5H)-one
Ref: Charles
Fehr & Iris Farris; Naphthalenone Derivative
with Powdery-Ionone Type Odors, United States
Patent Application 20080214419 (September 4,
2008)
|
|



