|
|
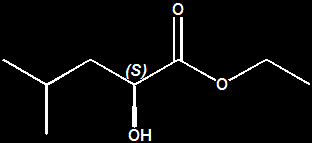
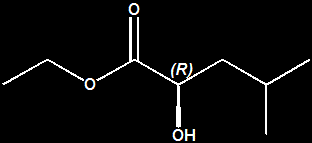
John C. Leffingwell, Ph.D. The Ethyl 2-hydroxy-4-methylpentanoates You must have
Java
installed to view the
molecular visualization on this
page Photo by permission of M. Roudintska - Art & Parfum |
|
|
c
c |
|
|
|
Ionones, Irones, Damascones & Structurally Related Odorants |
|
Copyright 2001-2002 - Leffingwell & Associates