|
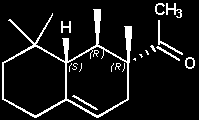
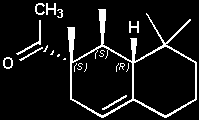
(+)-(1R,2R,8aS)-Arborone
- possesses an intense woody odor that was clean
and very pleasant.
Odor
Threshold - Comparative tests in Corey's
laboratory indicated that the odor threshold for
(+)-Arborone is 20 to 30 times lower than that
for (–)-Georgyone, the active enantiomer in
racemic Georgywood®. [Comparatively,
Frater, et. al., have reported an odor threshold
of 20 pg/L for the odor active (-)-Georgyone
enantiomer (Determined on the sniff-outlet of a
chiral GC-column with a panel of three
experienced fragrance chemists) and 5 pg/L for
racemic Arborone].
=
(+)-1-[(1R,2R,8aS)-1,2,8,8-tetramethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-yl]ethanone
Ref:
Ref: Sungwoo Hong and E. J. Corey,
Enantioselective Syntheses of Georgyone,
Arborone, and Structural Relatives. Relevance to
the Molecular-Level Understanding of Olfaction,
J. Am. Chem. Soc., 2006; 128(4) pp 1346 - 1352;
Georg Frater, Urs Muller and Fridtjof Schroder,
Synthesis and olfactory properties of
(-)-(1R,2S)-Georgywood, Tetrahedron: Asymmetry,
15, 3967–3972 (2004) ; See Cornelius
Nussbaumer, Georg Fráter, Philip Kraft,
(±)-1-[(1R*,2R*,8aS*)-1,2,3,5,6,7,8,8a-Octahydro-1,2,8,8-tetramethylnaphthalen-2-yl]ethan-1-one:
Isolation and Stereoselective Synthesis of a
Powerful Minor Constituent of the Perfumery
Synthetic Iso E Super®
, Helvetica
Chimica Acta, Volume 82, Issue 7, Date: July 7,
1999, Pages: 1016-1024 for the synthesis of the
racemic "Arborone" and odor evaluation &
threshold.
An outline of
Hong & Corey's synthesis can be found
HERE.
Iso E
Super®
is a trademark
of International Flavors &
Fragrances
|