|
|
John C. Leffingwell, Ph.D. The 2-Methyl-4-(5,5,6-trimethyl-2-norborny)cyclohexanones You must have
Java
installed to view the
molecular visualization on this
page |
|
|
|
|
||
|
|
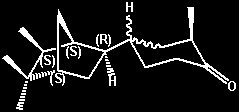
(-)-2-Methyl-4-(5,5,6-exo-trimethyl-2-endo-norborny)cyclohexanone
(liquid [axial methyl?] isomer) -
moderately strong urinous On a relative
basis the urinous intesity is about 40% that of
Theimers urinous ketone -
cis-4-(4-tert-Butylcyclohexyl)-4-methylpentan-2-one,
or about 4% that of (+)-Androstenone. Both a solid
isomer (with the cyclohexanone methyl group
presumably being equitorial) and a liquid isomer
(with the cyclohexanone methyl group presumably
being axial) were isolated and individually
evaluated for the urinous character. However the
situation appears more complex as either oientation
(axial or equitorial) can also exist in both
erythro and threo forms. Also referred to
as d-4(liquid) or d(-)-endo-4 (liquid) =
(-)-2-methyl-4-[(1R,2R,4R,6R)-5,5,6-trimethylbicyclo[2.2.1]hept-2-yl]cyclohexanone
(liquid isomer) Ref: Ernst
T. Theimer, Takao Yoshida, and Erich M. Klaiber,
Olfaction and Molecular Shape. Chirality as a
Requisite for Odor, J. Agric. Food Chem., Vol. 25,
No. 5, 1977, 1168-1177 |
||
|
|
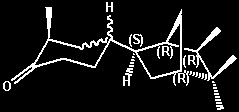
(-)-2-Methyl-4-(5,5,6-exo-trimethyl-2-endo-norborny)cyclohexanone
(solid [equitorial? methyl] isomer) -
urinous On a relative
basis the urinous intesity is about 10% that of
Theimers urinous ketone -
cis-4-(4-tert-Butylcyclohexyl)-4-methylpentan-2-one,
or about 1% that of (+)-Androstenone. Both a solid
isomer (with the cyclohexanone methyl group
presumably being equitorial) and a liquid isomer
(with the cyclohexanone methyl group presumably
being axial) were isolated and individually
evaluated for the urinous character. However the
situation appears more complex as either oientation
(axial or equitorial) can also exist in both
erythro and threo forms. Also referred to
as d-4(liquid) or d(-)-endo-4 (liquid) =
(-)-2-methyl-4-[(1R,2R,4R,6R)-5,5,6-trimethylbicyclo[2.2.1]hept-2-yl]cyclohexanone
(liquid isomer) Ref: Ernst
T. Theimer, Takao Yoshida, and Erich M. Klaiber,
Olfaction and Molecular Shape. Chirality as a
Requisite for Odor, J. Agric. Food Chem., Vol. 25,
No. 5, 1977, 1168-1177 |
|
|
Ionones, Irones, Damascones & Structurally Related Odorants |
|
Copyright 2001 - Leffingwell & Associates


-endo4_liquid.gif)
-endo-4_liquid.gif)
-endo4_solid.gif)
-endo-4_solid.gif)